Introduction. The morphology of stones has special importance. Stones consist of organic, inorganic and amorphous part. Many stones contain the amorphous organic substance, which makes up 2–3% of the weight of the matrix [1].
The factors that affect the formation of stones can be divided into two categories:
1. Those that are in the urine and form the crystalline nucleus, agglomeration, which includes proteins, salts, glycoprotein’s, phospholipids.
2. Changes in cell surface leading to crystal adhesion to epithelial cells [1, 3].
Proteins are believed to play a major role in stone formation because they have the potential to cause crystal-membrane interaction. Anti-inflammatory proteins such as myeloperoxidase and interleukin-6 initiate urolithiasis. Also, metabolic disorders hyperparathyroidism, hyperthyroidism, obesity, sarcoidosis, hypercalcemia; hyperuricosuria affects the occurrence of urolithiasis [8, 11]. Infections also affect the appearance of stones, especially infectious stones (urease-producing bacteria; Proteus, E.coli, Klebsiela [24, 26]. Renal stone formation is attributed to risk factors entering urolithiasis; crystal supersaturation, small citrate excretion, metabolic disorders, genetic predisposition [1, 2]. Urolithiasis is a disease of multifactorial etiology. The prevalence of urolithiasis in the urinary tract ranges from 1% to 20% [8]. In countries with high standards such as Sweden, Canada, USA, the prevalence of urinary tract stones is not high (> 10%). For some countries it ranges up to 37% [1]. Lithogenesis is a multifactorial disorder. In most European countries and the USA, upper urinary tract stones are found in 2–3% of the population. About 90% of stones are found in the upper parts of the urinary tract (calyx, pyelonephritis, and ureter) and only 10% are found in the urinary bladder [1, 9].
The chemical analysis of the morphopathology of urolithiasis will be done with the method of X-ray diffraction and infrared spectroscopy, where with these methods will be determined: the composition of uroliths (stones), nucleus, envelope, nucleus component and stone layer component, the prevalence of the nuclear component and the prevalence of the urolithic layer component. At the end of the research we will conclude about the morphopathology of urolithiasis and will propose preventive measures for the occurrence of urolithiasis in patients of the Republic of Kosovo.
Materials and methods. The research was conducted on our patients at the Urology Clinic, University Clinical Centre of Kosovo, in Pristine. The paper was prospective patients are of both genders and of all ages. The study protocol was approved by the Ethics Committee of the Faculty of Medicine in Pristine. All patients before participating in the study were informed about our research, and after receiving their consent we started the research. In this research we have included 102 patients. The most attacked age group, gender, will be determined. Patients who have been operated -102 patients, where the stones were extracted through methods: endoscopic surgery; URS (Ureterorenoscopy) with lithotripsy, and open surgery; pyeloliotomy, nephrolithotomy. After the intervention we took the stone and did the chemical analysis of the stones with the method: X-ray diffraction and infrared spectroscopy. This will determine the components of the stones and their types.
Statistical analysis
Data processing was done with the statistical package InStat 3. The obtained data were presented through tables and graphs. From the statistical parameters the structure index, arithmetic mean, and standard deviation were calculated. Qualitative data testing was done with X2-test or Fisher test. Quantitative data testing is done with T-test. Test verification was done with a reliability rate of 99.7% (p<0.01) and a reliability of 95% (p<0.05).
Results. According to the age group out of 102 patients included in the research, 4 or 3.9% are in the age group 0–9 years and 10–19 years, 12 or 11.8% are in the age group 20–29 years, 26 or 25.5% are of age group 30–39 years; 29 or 28.4% are in the age group 40–49 years; 10 or 9.8% are in the age group 50–59 years, 12 or 11.8% are in the age group 60–69 years and 5 or 4.9% are in the age group 70 and more.

The study included 102 patients with urolithiasis of whom 68 or 66.7% were male and 34 or 33.3% female. With X2-test we gained a difference with statistically significant significance in the number of cases by gender (X2 =11.3, P=0.001), (Fig. 2).
From 102 patients with stone at 60 or 58.8% the chemical component of the stones was Calcium oxalate monohydrate at 15 or 14.7% Calcium Phosphate, at 11 or 10.8% Magnesium ammonium phosphate, at 6 or 5.9% Calcium oxalate dihydrate, at 5 or 4.9% Uric acid, at 3 or 2.9% Cystinic, at one or 1.0% Amonium HydrogenUrate and 1 or 1.0% Brushite (Tab. 1 and Figure 3).
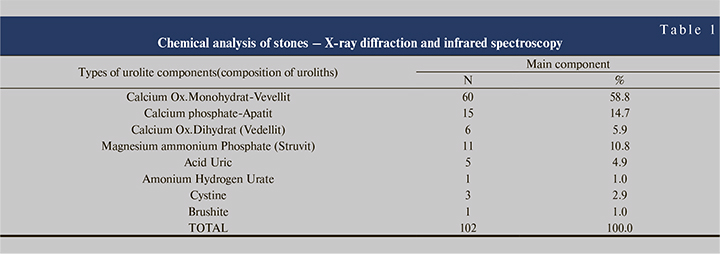
Out of 102 stone patients from this group, we have the group of children who are 8 cases or 7.84%. The chemical component of the stones had emerged in this composition; Calcium oxalate monohydrate (vevellit) in 3 cases (2.94%), Cystinic; 2 cases (1.96%), Calcium Ox. Dihydrate (vedellit); 1 case (0.98%) magnesium ammonium phosphate (Struvit); 1 case (0.98%) uric acid; 1 case or 0.98%.
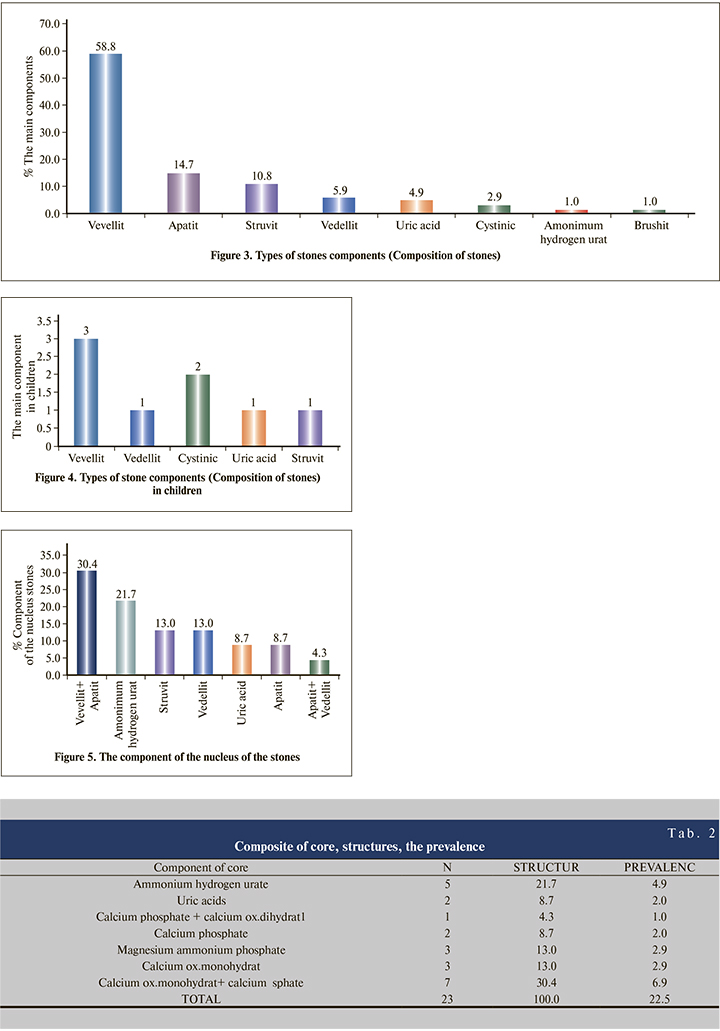
The core component in 30.4% of cases was Calcium oxalate monohydrate and Calcium phosphate (prevalence 6.9%), 21.7% of cases was Ammonium hydrogen urate (prevalence 4.9%), from 13.0% was Magnesium ammonium phosphate and Calcium oxalate monohydrate 2.9 (prevalence) %), from 8.7% was uric acid and Calcium phosphate (prevalence 2.0%) and 4.3% was Calcium phosphate with Calcium oxalate dihydrate (prevalence 1.0%), (Tab. 2).
Discussion. The most sophisticated methods are used today for the chemical analysis of stones; X-ray diffraction and infrared spectroscopy. X-ray diffraction has an advantage over other methods because it analyzes the crystalline composition of the stones. Infrared spectroscopy is more sophisticated to identify non-crystalline materials, including amorphous substances and fats.
The nucleus can be interpreted as the center of growth but this is not necessarily the geometric center of the stone. Thin-cut studies by Seyfarth et al., Performed by scanning electronic microscope, suggest that uroliths pass through one or two primary growth centers, often coexisting with a number of irregularly distributed secondary growth centers.
They are later responsible for the frequency of irregular stone shapes and surfaces. Central region according to Schneider, Han and Eismann: growth centers can be divided into several types: a). the central region, which is completely isotropic, while its structure is unknown, while the isotropic crystalline matter is irregular in configuration resembling cobblestones, and) the center is characterized by a cavitary formation. Studies by Schneider and Seyfarth on the nucleus conclude that the adsorption of material by individual crystals plays a sub-ordinating role in the mechanism of stone formation.
Aggregation in the central area is characterized to some degree by intermicelar crystallization or by the presence of an adequate matrix. Later, a part leads to the excretion of colloids or inorganic crystals (Seyfartth and Schnaider).
Damaged cellular elements and blood clots may also represent the starting point of lithogenesis.
On the other hand, other authors follow the theory of crystallization, considering the formation from crystals or the aggregation of crystals as a case (seed for planting), which may be a precursor of uroliths, which are then mechanically fixed epitaxically or hydrodynamic, within gelatin everywhere in the urinary system.
These entities later can grow subconsciously and become the starting point for stone formation. An impressive aspect of the way uroliths aggregate, brought to us by Sutor and Wooley, is the linear growth property. Here emerges a special relationship between the axis of the crystals and the radial growth of the crystals, which later show a marked preference for perpendicular growth within the individual strata (annual annular structure).
In our research where the chemical analysis of stones was done, in 102 patients with stones located in; the kidney, ureter and bladder. In our research with urolithiasis we have both genders, of which 68 or 66.7% were male and 34 or 33.3% female. With X2-test we gained a difference with statistically significant significance in the number of cases by gender (X2=11.3, P=0.001).
From the chemical analysis of the stones with the method of X-ray diffraction and infrared spectroscopy shows that the most attacked age is (40-49 years old). After the intervention - removal of stones, chemical analysis of stones is performed with the above mentioned methods. Where the stones have this composition: Calcium oxalate monohydrate (vedellit) constitutes the largest number of patients with 60 cases (58.8%). Calcium Phosphate (Apatit) we have 15 cases (14.7%), Magnesium amonium phosphate (Struvit) with 11 cases (10.78%), then Calcium oxalate dehydrate (Vedellit) with 6 cases (5.88%). In our material we have also encountered stones cystine in 3 cases (2.94%) which are quite rare in other authors. These stones appear due to genetic disorders. Our research is dominated by calcium oxalate stones, followed by phosphate stones – infectious stones (apathy and struvitis).
From the chemical analysis of the stones in our research, it turns out that the core of flat stones contains Ammonium Hydrogen Urate. The core component in 30.4% of cases was Calcium oxalate monohydrate and Calcium phosphate (prevalence 6.9%), 21.7% of cases was Ammonium hydrogen urate (prevalence 4.9%), from 13.0% was Magnesium ammonium phosphate and Calcium oxalate monohydrate 2.9 (prevalence) %), from 8.7% was uric acid and Calcium phosphate (prevalence 2.0%) and 4.3% was Calcium phosphate with Calcium oxalate dihydrate (prevalence 1.0%. In 28.6% of cases the layer component was Calcium oxalate monohydrate and Calcium phosphate (prevalence 3.9%), in 21.4% it was uric acid (prevalence 2.9%), and from 14.3% it was Calcium phosphate and Magnesium ammonium phosphate (prevalence from 2.0%). From this research we can say that Kosovo based on the analysis of stones can be ranked in countries with high standards, in terms of stone components.
From the chemical analysis of the stones and the table we see that Calcium oxalates make up 63.96% of the stones in our material (Vevellit 58.8% and Vedellit 5.88%), so Calcium Oxalat monohydrate is the dominant component of both genders. Then Magnesium Ammonium Phosphate is common in men and stones in the lower urinary tract (infectious stones-Struvit), especially in patients with subvesical obstruction; prostate hyperplasia, urethral strictures, while in women dominate Calcium Fosfat (apathy), which occurs most often in the upper urinary tract of women.
We can compare our data with those of other authors in different countries. We do not have major differences with the authors: Schanajder, Rebentich, where our material is dominated by Calcium oxalates; vevellit with 58%, and vedellit 5.9%, in Schneider we have 58.6%, vevellit, and 18% vedellit, while in Rebentich we have 56.4% vedellit and 15% vedellit.
The difference in infectious stones is that we are dominated by Apatite 14.7% and struvites with 10.78%, while Schneider apatite constitutes 3.5%, while struvites 5.1%, Rebentich apatite 3.62% and struvites 5.87%. So in our material dominate more infectious stones, which are in higher numbers than other authors. In our material we have 3 cases (2.94%) with cystinic stones, until these stones are not found in the above authors. Cystinic stones are stones that are caused by genetic disorders and have a high recurrence.
Pathomorphological analysis of urolithiasis is approximate to the data of other authors, where we are dominated by Calcium Oxalate stones.
In the upper urinare tract are mainly Ca.Ox stones, component of Vevellit and Vedellit, while in the lower tract Apatite and struvite (infectious stones) dominate, usually in men due to subvesical obstructions. The pathogenesis of stones to this day is a problem in itself; there are still no explanations about the pathogenesis of urolithiasis.
By knowing the pathomorphology of the stones, prophylactic measures can be taken and their recurrence can be prevented. In this way patients are provided with the best quality of life and at the same time prevent chronic kidney damage from stones.
Conclusion. X-ray diffractometry and infrared spectroscopy are the most sophisticated methods for chemical analysis of stone composition. X-ray diffractometry analyzes the crystalline components of stones, while infrared spectroscopy serves to identify non-crystalline materials, including amorphous substances and fats. Urolithiasis involves both genders, more males than females, age mostly affected is ( 40–49 years old).In our research we have this composition of stones; Calcium oxalate monohydrate (vevellit) 58.8%), Calcium Phosphate (Apatit) 14.7%), Magnesium ammonium phosphate (Struvit) 10.78%, Calcium oxalate dehydrate (Vedellit) 5.88%, cystine stones 2.94%, which are quite rare in other authors, these stones appear due to genetic disorders. From 102 patients, we have the group of children which are 8 cases or 7.84%. Chemical composition consists of: Calcium oxalate monohydrate (vevellit) 2.94%, Cysteine; 1.96%, Calcium Oxalat Dihydrate (vedellit); 0.98%, Magnesium ammonium phosphate (Struvit); 0.98%, uric acid; 0.98%. From the chemical analysis of stones, we have the result that the most common analysis of stone nucleus is Ammonium Hydrogen Urate. Stone analysis can be ranked in countries with high standard. The pathogenesis of stones to this day is a problem in itself, as there are still no explanations about the pathogenesis of urolithiasis. By knowing the pathomorphology of the stones, prophylactic measures can be taken and their recurrence can be prevented.



