Введение. Последние годы ознаменованы неуклонным ростом количества мужчин, обращающихся к урологу по поводу различных расстройств мочеиспускания: так называемых симптомов нижних мочевыводящих путей (СНМП). Учитывая негативное влиянием этого состояния на качество жизни пациента, своевременная диагностика и адекватный выбор урологом лечебной тактики имеют большое значение. Симптомы нижних мочевыводящих путей – собирательное понятие, включающее большое разнообразие полиэтиологичных симптомов, характерных для ряда заболеваний органов малого таза. Выбор оптимального режима медикаментозной терапии среди многообразия современных методов лечения СНМП представляет собой сложную задачу, решение которой возможно только с использованием междисциплинарного подхода [1, 2].
Доброкачественная гиперплазия предстательной железы (ДГПЖ) – наиболее частая причина СНМП у мужчин среднего, пожилого и старческого возраста. С возрастом распространенность ДГПЖ увеличивается, достигая 90% к 90 годам. Ее развитие и прогрессирование связывают с идиопатической пролиферацией стромальных и гландулярных структур периуретральной зоны предстательной железы [3, 4].
У каждого третьего перешагнувшего 50-летний рубеж мужчины ДГПЖ служит причиной развития умеренных или выраженных СНМП. Этиология гиперплазии предстательной железы изучена недостаточно. Существует несколько теорий, призванных объяснить причины увеличения простаты при ДГПЖ. Поскольку предстательная железа является гормонально зависимым органом, в основе всех теорий лежит идея гормональной перестройки организма, которая наступает у мужчин в 50–60-летнем возрасте [5, 6].
Поддержание нормального функционирования и взаимодействия клеток в простате осуществляется массой биологически активных веществ – пептидов, регулирующих рост и размножение клеток путем ингибирующего и стимулирующего воздействия (факторы роста). Синтез этих факторов происходит преимущественно в строме, а влияют они как на стромальные клетки, так и на эпителий. Для роста простаты наибольшее значение имеют стимулирующие факторы: эпидермальный и инсулиноподобные факторы роста, трансформирующие факторы роста α и β, основной фактор роста фибробластов и кератиноцитов. Взаимодействие этих факторов в простате – сложный и не до конца изученный процесс. Дисбаланс указанных пептидов с преобладанием, стимулирующих рост простаты, приводит к развитию ДГПЖ. Одним из дополнительных факторов, увеличивающих активность пролиферации клеток, является избыток эстрогенов. Апоптоз – процесс запрограммированной гибели клетки. При нарушении баланса факторов, влияющих на пролиферацию клеток и на их гибель, может снижаться активность апоптоза. Многие исследователи считают ДГПЖ болезнью, связанной не с избыточным размножением клеток, а с их недостаточной гибелью.
Нарушение баланса андрогенов и эстрогенов, приводящее к замедлению процессов клеточной смерти (апоптоза), в сочетании с бурным ростом стволовых клеток обусловливает увеличение размеров предстательной железы. Мышечная ткань шейки мочевого пузыря, уретры, сфинктеров, простаты обеспечивает развитие динамического компонента обструкции, являющегося итогом повышения активности симпатической нервной системы, поддерживающей гипертонус вышеперечисленных структур нижних мочевыводящих путей [7–9].
Целью исследования стало получение данных по эффективности и безопасности применения препарата Афалаза для лечения СНМП у пациентов с ДГПЖ без предшествовавшей терапии.
Материалы и методы. В девяти московских урологических центрах проведено проспективное клиническое многоцентровое исследование эффективности применения препарата Афалаза в лечении СНМП у пациентов с ДГПЖ без предшествовавшей терапии.
В исследовании приняли участие 80 пациентов, соответствовавших критериям включения:
- пациенты мужского пола в возрасте ≥40 лет;
- подтвержденная при трансректальном УЗИ (ТРУЗИ) доброкачественная гиперплазия предстательной железы с объемом предстательной железы ≥30 см3;
- оценка по шкале IPSS 8–19 баллов;
- стабильные интимные отношения с женщиной-партнершей на протяжении как минимум последних 6 мес., которые планировалось продолжить в течение всего исследования.
Критерии невключения:
- показания к хирургическому лечению ДГПЖ;
- в анамнезе прием лекарственных препаратов для лечения ДГПЖ;
- наличие в анамнезе инвазивных методов лечения ДГПЖ, включая трансуретральную резекцию простаты (ТУРП), термо-, микроволновую терапию, трансуретральную игольчатую аблацию, стентирование и др.;
- запланированные на время участия пациента в исследовании инвазивные методы лечения ДГПЖ, включая ТУРП, термо-, микроволновую терапию, трансуретральную игольчатую аблацию, стентирование и др.;
- острая задержка мочеиспускания в течение 3 мес. до включения в исследование.
Cредний возраст пациентов составил 57,7±7,3 (95% доверительный интервал [ДИ] – 56,1–59,4) года, средний уровень простатспецифического антигена (ПСА) – 1,7±1,1 (95% ДИ – 1,5–2,0) нг/мл.
Дизайн исследования предусматривал девять визитов (табл. 1). На Визите 1 проводили сбор жалоб, анамнеза, объективный осмотр, ТРУЗИ (определение объема предстательной железы, объема остаточной мочи), урофлоуметрию (определение максимальной скорости потока мочи – Qmax), определение содержания ПСА, заполнение опросников IPSS, QoL, МИЭФ-5. При соответствии критериям включения и отсутствии критериев невключения пациенту выдавали препарат Афалаза.

Схема исследуемой терапии препаратом: по 2 таблетки под язык до полного растворения, не во время приема пищи, 2 раза в день – утром и вечером.
На последующих восьми визитах пациенты проходили предусмотренный дизайном исследования комплекс обследования (табл. 1). На заключительном Визите 9 (30-я неделя±7 дней) наряду с этим пациенты заполняли опросник удовлетворенности лечением.
За 3 мес. до включения в исследование, а также в ходе исследования (с момента подписания информированного согласия и начала скрининга) пациенты не могли применять любую терапию, способную повлиять на течение ДГПЖ (в том числе α1-адреноблокаторы, ингибиторы 5α-редуктазы, экстракт сливы африканской коры, экстракт плодов пальмы ползучей, мепартрицин). Было запрещено принимать препараты для лечения нарушений эрекции, препараты на основе высоких разведений антител к эндотелиальной NO-синтазе и ПСА (Импаза, Афала, Диваза); модуляторы синтеза оксида азота (в том числе небиволол), донаторы оксида азота (нитраты и нитратоподобные средства пролонгированного действия, за исключением нитратов по требованию при приступах стенокардии), любые незарегистрированные лекарственные препараты и/или вакцины; препараты, при применении которых у пациента ранее отмечались аллергические реакции. В течение периода исследования пациентам был разрешен прием препаратов для терапии сопутствующих заболеваний.
В состав лекарственного препарата Афалаза входят аффинно очищенные антитела к эндотелиальной NO-синтазе (анти-eNOS) и аффинно очищенные антитела к ПСА (анти-PSA). Данные компоненты наносят на лактозу в виде смеси трех активных водно-спиртовых субстанций, разведенных соответственно в 10012, 10030, 100200 раз.
Анти-eNOS способствуют увеличению скорости кровотока в сосудах полового члена и предстательной железы; оказывают защитное действие на эндотелий (способствуют снижению реактивности сосудов, уменьшению сосудистого спазма и улучшению периферической микроциркуляции).
Анти-PSA модифицируют функциональную активность эндогенного ПСА, нарушающуюся на фоне ДГПЖ, что сопровождается усилением регуляторного влияния данного антигена на функциональные и метаболические процессы в ткани предстательной железы.
Обработанные таким образом антитела, в отличие от нативных антител, способны модифицировать активность своей мишени, а не связывать ее и тем самым блокировать активность. Данное свойство позволяет использовать их для воздействия практически на любые фармакологические мишени в организме [10]. Экспериментально и клинически показано, что препараты, приготовленные по такой технологии, по эффективности не уступают стандартным препаратам, используемым для лечения гриппа, сахарного диабета, гиперплазии предстательной железы [11–18].
Основным критерием эффективности препарата Афалаза послужило изменение суммарного балла по шкале IPSS через 30 нед. ежедневного приема.
Вторичными критериями эффективности проводимого лечения стали следующие параметры:
- динамика балла вопросника качества жизни (QoL);
- изменение эректильной функции (динамика балла опросника МИЭФ-5);
- динамика объема предстательной железы;
- динамика показателя Qmax;
- изменение объема остаточной мочи;
- удовлетворенность пациента лечением (средний балл по вопросам «эффективность лечения», «безопасность лечения», доля пациентов, рекомендовавших лечение препаратом Афалаза другому пациенту).
Обработку данных и статистические расчеты производили с использованием статистического пакета SAS-9 (лицензиат ООО НПФ «“Материа Медика Холдинг”», № 70100045). Исследуемые непрерывные/интервальные переменные представлены в виде среднего стандартного отклонения, а также 95% ДИ. Категориальные переменные представлены в виде частотных таблиц отдельно по визитам, в виде абсолютного и относительного числа случаев/пациентов. Для оценки различий непрерывных переменных, полученных в одной группе на двух разных визитах использовали t-критерий Стьюдента для парных выборок. Для выполнения вышеописанного статистического анализа применяли процедуры SAS: FREQ, MEANS – вычисление описательных статистик; TTEST – t-критерий Стьюдента со всеми его модификациями.
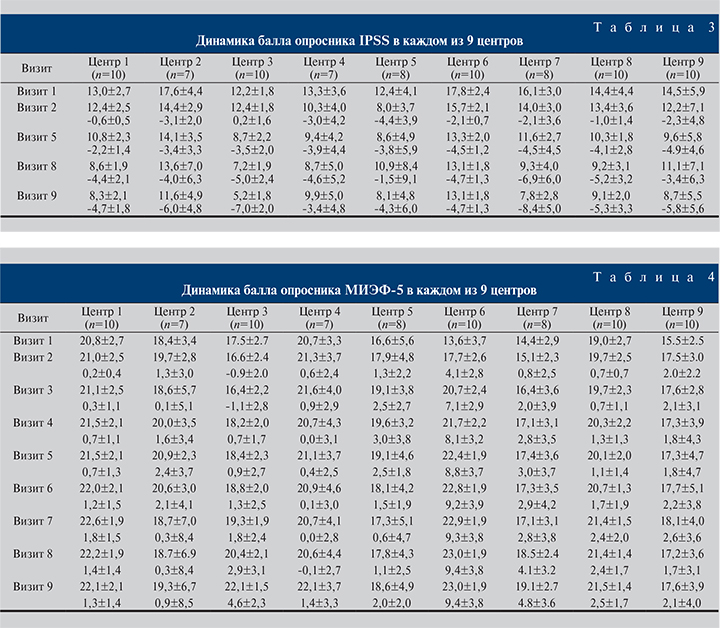
Результаты. Динамика оцениваемых параметров представлена в табл. 2–5. Все изменения были статистически значимыми. По окончании 30-недельного приема препарата констатировали снижение суммарного балла IPSS на 5,5 баллов (37,9%) – с 14,5±4,0 до 9,0±4,1. Обращает на себя внимание постепенное и плавное снижение показателя на протяжении всего исследования (табл. 3).
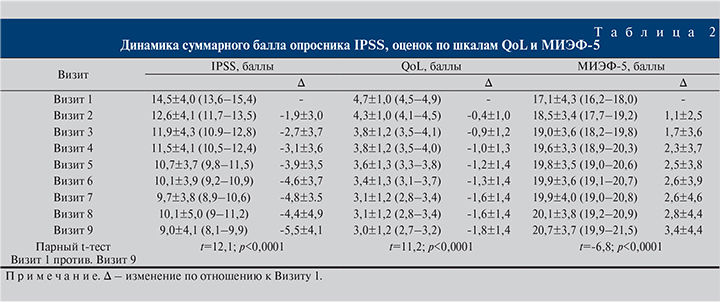
Один из вторичных критериев оценки эффективности, балл опросника QoL (табл. 2), в целом снизился на 1,8 (38,3%) – с 4,7±1,0 до 3,0±1,2, что говорило об улучшении качества жизни пациентов на фоне приема исследуемого препарата.
Важной частью исследования была оценка качества эректильной функции согласно валидизированному опроснику МИЭФ-5 (табл. 2). Спустя 30 нед. терапии пациенты отметили улучшение эректильной функции, о чем свидетельствовало повышение суммарного балла опросника МИЭФ-5 на 3,4±4,4 (19,9%) – с 17,1±4,3 до 20,7±3,7 (табл. 4).
Прием препарата Афалаза пациентами всех девяти центров сопровождался увеличением показателя. Qmax, который вырос на 3,6±4,9 мл/с (28,3%), составив к концу периода наблюдения 16,4±5,7 мл/с (табл. 5).
По результатам проведенного исследования также констатировали положительную динамику показателей объема предстательной железы и объема остаточной мочи. Так, объем предстательной железы уменьшился на 2,2±4,5 см3 (5,15%), составив к концу наблюдения 41,0±9,8 см3, объем остаточной мочи снизился на 8,3±21,6 мл (31,9%) и составил 17,7±24,2 против 26,0±25,3 мл на Визите 1 (табл. 5).

За весь период исследования нежелательных явлений, связанных с приемом указанного препарата, зарегистрировано не было. Все пациенты успешно завершили участие в исследовании. Ни в одном из центров не было ни одного случая досрочного завершения исследования.
Обсуждение. Терапия препаратом Афалаза на протяжении 30 нед. уменьшила выраженность СНМП, улучшила эректильную функцию, привела к увеличению Qmax и снижению объема предстательной железы у мужчин с ДГПЖ. Облегчение СНМП и улучшение эректильной функции проявлялись в первые три недели лечения, положительная динамика сохранялась до конца исследования. Улучшение IPSS, Qmax и объема простаты достигло максимума в конце терапии, но не вышло на плато. Для оценки риска прогрессирования ДГПЖ были рассмотрены проверенные факторы риска, включая общий уровень IPSS, Qmax, объем простаты [19, 20]. Применение препарата Афалаза привело к значительному снижению общей суммы симптомов прогрессирования ДГПЖ.
Сложно сравнивать результаты этого исследования с любыми другими продолжительными исследованиями, в ходе которых изучалось влияние длительной медикаментозной терапии на СНМП/ДГПЖ и прогрессирование заболевания из-за присутствия в них групп сравнения с ингибиторами 5α-редуктазы и α-адреноблокаторами. Так, лечение финастеридом на протяжении 4 лет в исследовании PLESS повысило оценку симптомов на 2,6 балла (по сравнению с 1,0 для плацебо в соответствии с индексом симптомов Американской урологической ассоциации; p<0,001) [21]. Исследование MTOPS продемонстрировало, что финастерид снижает риск развития симптомов (определяемый как увеличение индекса симптомов американской урологической ассоциации ≥4 баллов) на 30% по сравнению с плацебо (р=0,016) [22].
Ингибиторы 5α-редуктазы оказывают антиандрогенное действие, ингибируя переход тестостерона в дигидротестостерон, что приводит к снижению синтеза белка и инволюции ткани простаты. Афалаза имеет совершенно другой механизм действия. Фармакологическая активность препарата связана с модулирующим воздействием на ПСА и эндогенную NO-синтазу через изменение их конформации. Простатспецифический антиген подавляет рост и миграцию эндотелиальных клеток путем ингибирования реакции эндотелиальных клеток на фактор роста двух фибробластов и фактор роста эндотелия сосудов. Эндогенная NO-синтаза поддерживает расширение кровеносных сосудов, контролирует кровяное давление и обладает множеством других вазопротекторных эффектов [23]. Предполагается, что синергическое действие анти-eNOS и анти-PSA улучшает метаболизм и васкуляризацию предстательной железы, оказывает антипролиферативное и противовоспалительное действия, что способствует улучшению функции мочевыводящих путей. Эти эффекты были продемонстрированы in vivo и в клинических испытаниях для каждого конкретного компонента Афалазы [11, 13, 24]. Сравнение результатов исследования с данными международных многоцентровых клинических испытаний, в которых изучалась эффективность комбинированного (ингибиторы 5альфа редуктазы (5АРИ)+α-адреноблокатор) или монокомпонентного (5АРИ/α-адреноблокатор) лечения при ДГПЖ, подчеркивает положительный эффект терапии Афалазой. Эффективное и безопасное воздействие, которое Афалаза оказывает на гиперплазированную предстательную железу, реализуется в уменьшении объема простаты и облегчении СНМП [13]. Совокупное улучшение симптомов СНМП/ДГПЖ, уменьшение объема простаты и объема остаточной мочи, повышение Qmax без достижения плато в течение 30 нед. указывают на то, что длительное использование Афалазы может препятствовать прогрессированию ДГПЖ.
Отсутствие пациентов с тяжелыми СНМП, тяжелыми сопутствующими заболеваниями в возрасте старше 70 лет, относительно короткая продолжительность и небольшой размер выборки можно рассматривать как ограничение проведенного исследования.
Заключение. Препарат Афалаза продемонстрировал хорошую эффективность и безопасность для пациентов с ДГПЖ без предшествовавшей лекарственной терапии. Баланс эффективности и безопасности выгодно отличает этот препарат от других, используемых для лечения СНМП/ДГПЖ медикаментозных средств. Общее снижения балла шкалы IPSS доказывает значимое уменьшение выраженности симптомов и указывает на возможность длительной терапии препаратом Афалаза. Это предположение требует подтверждения в дополнительных исследованиях и в больших популяциях.
Полученные результаты должны быть учтены специалистами при выборе лекарственной терапии пациентов с «начальными проявлениями симптомов лечения аденомы». Важен тот факт, что в московскую программу вошли пациенты, ранее не получавшие никакой терапии по поводу СНМП/ДГПЖ. Известно, что мужчины в Российской Федерации «не любят» обращаться к врачам и зачастую их первое обращение к урологу происходит несвоевременно, но полученные нами данные демонстрируют, что препарат Афалаза может быть применен как препарат первой линии лечения пациентов с СНМП/ДГПЖ. Важно отметить эффективность Афалазы в отношении эректильной функции у наших пациентов, что подтверждено увеличением балла по шкале МИЭФ-5.



