Для принятия решения о выполнении биопсии простаты при первичном обследовании с целью выявления рака предстательной железы (РПЖ) необходимо учитывать ряд факторов, таких как возраст, семейный анамнез пациента, данные пальцевого ректального исследования (ПРИ), уровень общего простатспецифического антигена (ПСА), соматический статус и пр. Использование такого подхода привело к увеличению выявляемости РПЖ и снижению смертности от данного заболевания. По данным Института измерений и оценки здоровья населения (IHME), в 2016 г. среди мужского населения было зарегистрировано 5,7 млн случаев РПЖ, который занимает 3-е место по распространенности среди онкологических заболеваний, уступая только раку молочной железы и колоректальному раку (см. рисунок). В 2017 г. в США было выявлено 161 360 случаев РПЖ, в 6 из 10 случаев заболевание выявляют у мужчин в возрасте 62 лет и старше, редко у мужчин моложе 40 лет [1]. Из представленных данных очевидно, что РПЖ редко становится причиной смерти, однако летальность при РПЖ прямо пропорциональна стадии заболевания.

В 1989 г. K. Hodge et al. с развитием технологий обосновали эффективность использования секстантного метода, таким образом исследователями были значительно расширены протоколы биопсии предстательной железы [2]. Согласно данным [3], частота выявления РПЖ при стандартной биопсии простаты (сБП) под контролем трансректального УЗИ (ТРУЗИ) составила 22,6% [3]. Стоит отметить, что частота ложноотрицательных результатов секстантной биопсии составила 49% [4]. Позже специалисты пришли к вывод, согласно которому мультифокальная биопсия из 10–12 точек с латеральным направлением вколов по безопасности не уступает биопсии предстательной железы из 6 точек, а по эффективности даже превосходит ее, повышая показатель выявляемости РПЖ на 24–38% [5]. С тех пор техника выполнения биопсии простаты коренным образом не изменилась. Согласно рекомендациям Европейской ассоциации урологов (ЕАУ), в 2019 г. «золотым» стандартом выявления РПЖ остается мультифокальная биопсия под контролем ультразвука – сБП. Доступ к выполнению сБП выбирается на усмотрение врача. Преимущества метода – в его стоимости, возможности амбулаторного выполнения, достаточной изученности метода, безопасности [6]. К основным недостаткам метода относятся высокая частота ложноотрицательных результатов (около 30%), гипердиагностика (выявление клинически незначимого рака) и как следствие – излишнее лечение РПЖ с высокой вероятностью ухудшения качества жизни пациента. К сожалению, формирование групп активного наблюдения пациентов низкого риска не устранило недостатки биопсии под УЗ-контролем. Так, M. Ramírez-Backhaus et al. [7] по результатам патоморфологического исследования предстательной железы после радикальной простатэктомии (РПЭ) у пациентов группы активного наблюдения продемонстрировали, что неблагоприятная патологическая картина (сумма баллов Глисона 7 или стадия pT3) наблюдалась в 13,1–42,4% случаев. Ввиду имеющихся недостатков в точности диагностики РПЖ в конце 1980-х гг. предстательную железу стали исследовать при помощи МРТ для определения стадии заболевания, оценки степени вовлечения капсулы железы и выявления поражения семенных пузырьков [8]. С разработкой протокола Prostate Imaging Reporting and Data System version 1 (Pi-RADS v1) появилась возможность прецизионно оценивать ткань предстательной железы с целью определения участков, подозрительных на рак [9, 10]. В 2015 г. была представлена усовершенствованная версия протокола – Prostate Imaging Reporting and Data System version 2 (Pi-RADS v2), которая по сей день успешно используется радиологами и урорадиологами всего мира в качестве инструмента диагностики РПЖ [11]. Так, по данным S. Polanec et al. [12], обе версии превосходны, однако их диагностическая эффективность различна. А именно Pi-RADS v1 является предпочтительным методом оценки транзиторной зоны простаты, тогда как Pi-RADS v2 лучше определяет области интереса в периферической зоне предстательной железы. В 2017 г. авторы исследования PROMIS продемонстрировали, что использование мультипараметрической МРТ (мпМРТ) может позволить 27% пациентов избежать первичной биопсии и диагностировать на 5% меньше клинически незначимого РПЖ, а МР-фьюжн-биопсия под контролем ТРУЗИ способна выявить на 18% больше клинически значимого РПЖ по сравнению с сБП [13].
В широком смысле под термином «фьюжн-биопсия» подразумевают взятие материала из участков, подозрительных на РПЖ, при использовании какого-либо метода визуализации (МРТ, эластографии, УЗИ и пр.). Специалисты продолжают дискуссию об определении наиболее подходящей группы пациентов, которым безусловно показано выполнение таргетной биопсии предстательной железы. По мнению специалистов National Comprehensive Cancer network (NCCN), слишком рано рекомендовать выполнение таргетной биопсии всем пациентам при подозрении на РПЖ в повседневной практике специалиста. Напротив, подход использования мпМРТ до биопсии у всех мужчин, подходящих для активного лечения, недавно был одобрен Национальной системой здравоохранения Англии (NHS England) [14]. По данным NCCN и EAУ, при отрицательной биопсии в анамнезе пациентам с клиническим подозрением на РПЖ рекомендовано выполнение мпМРТ [15]. Таким образом, показания к выполнению таргетной биопсии под контролем МРТ постепенно расширяются, а эффективность повышается с увеличением числа исследований. Однако с учетом высокой стоимости МРТ-диагностики РПЖ специалисты пребывают в поисках более доступного и не менее эффективного метода. Таким методом является гистосканинг. При выполнении таргетной биопсии под контролем гистосканинга частота выявления РПЖ, по данным различных авторов, варьируется от 38 до 46% [16].
Таким образом, уже сейчас ясно, что дополнительные прицельные ориентиры при выполнении биопсии предстательной железы под УЗ-контролем повышают шансы обнаружения значимого заболевания и снижают вероятность выявления клинически незначимого РПЖ, тем самым улучшая качество диагностического поиска.
В данной работе под термином «точность биопсии» подразумевают процент случаев, когда на основании фьюжн-биопсии в подозрительном участке выявлены злокачественные клетки.
Материалы и методы. Для написания статьи были использованы базы данных MEDLINE/PubMed/Web of Science/, научной электронной библиотеки, Российской государственной библиотеки, где выполняли поиск научных работ, посвященных сравнению таргетных методик биопсии предстательной железы с сБП под УЗ-контролем. Для поиска научных работ в базе данных PubMed/Medline использовали следующие запросы: (prostate cancer OR prostate adenocarcinoma) AND (MRI or magnetic resonance) AND (targeted biopsy); (prostate cancer OR prostate adenocarcinoma) AND (PHS or Histoscanning) AND (targeted biopsy) и (prostate cancer OR prostate adenocarcinoma) AND (MRI or magnetic resonance) AND (targeted biopsy) AND (cognitive registration). В базе данных электронной библиотеки eLirary отобраны научные статьи по запросам: таргетная биопсия простаты/предстательной железы, гистосканирование простаты/предстательной железы, histoscanning, когнитивная биопсия простаты/предстательной железы, биопсия простаты/предстательной железы визуальное наведение. В результате были отобраны только те работы, в которых сравнивалась каждая методика таргетной биопсии предстательной железы с сБП, а также работы, в которых проводили сравнение между собой методики таргетной МР-биопсии (когнитивная c аппаратной фьюжн) и c биопсией под контролем гистосканинга. Причем одним из важных критериев было наличие четкой контрольной группы в каждом случае и результатов для каждой отдельной группы. Данные мужчин с отрицательными результатами МРТ, которые перенесли сБП, тоже были учтены.
Результаты. В ходе подготовки к анализу было отобрано 672 публикации, из них в анализ включено 25 оригинальных научных работ, в которых были опубликованы данные 4634 пациентов. Были выделены группы сравнения различных методик выполнения фьюжн-биопсии простаты.
Когнитивная МР-фьюжн-биопсия по сравнению с сБП
Все исследования, связанные с таргетной биопсией предстательной железы с применением МРТ, многие авторы рекомендуют подвергать определенным критериям оценки на основании руководств. Подвергаются оценке исследования, посвященные таргетной биопсии под контролем МРТ. С этой целью в 2013 г. был организован комитет для определения стандартов публикации исследований на тему МР-фьюжн-биопсии предстательной железы (START).
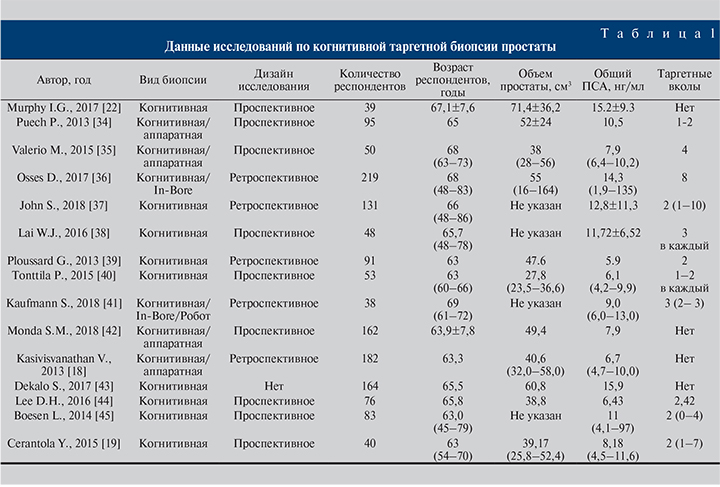
В качестве руководства для оценки публикаций на тему таргтеной биопсии простаты при помощи МРТ можно считать исследование C. M. Moore et al., которые определили самые строгие рекомендации: каждый проанализированный биоптат должен быть описан морфологом отдельно (в результате системной биопсии и таргетной) с использованием баллов Глисона и указанием максимальной длины биоптата; частоты выявления клинически значимого и незначимого рака предстательной железы при сБП и тБП; наличия биопсии в анамнезе; метода оценки МРТ; опыта специалиста лучевой диагностики и техники регистрации изображений [17]. Одной из прогрессивных научных работ по сравнению МР-фьюжн-биопсии предстательной железы и сБП стала статья, опубликованная в 2018 г. V. Kasivisvanathan et al., о результатах исследования PRECISION. Согласно опубликованным данным, клинически значимый рак обнаружен у 38 и 26% пациентов при выполнении МР-фьюжн-биопсии и сБП соответственно (+12; 95% CI от 4 до 20, р=0,005), клинически незначимый РПЖ был выявлен в 9 и 22% случаев соответственно (-13; 95% CI от -19 до -7; р<0,01). Авторами сделан вывод, согласно которому предварительная оценка риска при помощи МРТ с последующей биопсией подозрительных участков, ориентированной на МР-изображения, значительно превосходит сБП у пациентов без биопсии в анамнезе с подозрением на РПЖ [18]. Точность выявления, стадирование и определение степени злокачественности образования – основные показатели, определяющие эффективность метода. Схожие выводы продемонстрировали Y. Cerantola et al. [19], опубликовав данные обследования 40 пациентов. Всего 102 столбика биопсии из истинно положительных областей интереса по МРТ были доступны для конечного анализа. Стоит пояснить, что истинно положительными являются подозрительные участки на РПЖ по данным изображений, наличие злокачественных клеток, подтвержденных патоморфологическим исследованием после РПЭ. Таким образом, когнитивная МР-таргетная биопсия простаты достигла цели в 82% случаев (84/102). На снижение точности влияло расположение подозрительных участков в передней зоне (р=0,01). В другом исследовании [20], посвященном определению эффективности когнитивной таргетной биопсии простаты, в котором представлена наибольшая выборка пациентов (182), оценку изображений проводили по протоколу Pi-RADS v1, по результатам которой частота выявления РПЖ составила 57%.
Опираясь на данные ранее упомянутого исследования [19], I. G. Murphy et al. [21] в своей работе проверили способность когнитивной МР-фьюжн-биопсии обнаруживать РПЖ передней локализации в когорте пациентов с отрицательной биопсией в анамнезе и выяснили, что у 46,2% пациентов аденокарцинома была выявлена в передней зоне простаты. Клинически значимый рак был выявлен у 13 (33,3%) пациентов. Баллы по системе Pi-RADS и размер подозрительных участков по МРТ были больше у пациентов с положительными таргетными биоптатами. Таким образом, точность фьюжн-биопсии при баллах Pi-RADS, равных 5, 4 и 3, составила 90, 33 и 29% соответственно (p<0,05). В целом на основании значения Pi-RADS можно было прогнозировать наличие РПЖ по данным биопсии простаты. Y. Watanabe et al. [22] продемонстрировали ценность режима ADC в определении подозрительных участков для выполнения таргетной биопсии. В результате точность биопсии при использовании ADC составила 70,1%.
Авторы исследований (табл. 1) едины во мнении, будто когнитивная таргетная биопсия предстательной железы при среднем уровне общего ПСА 9,6 (4,7–15,2) нг/мл, среднем возрасте пациента 61,2 (63–69) года вне зависимости от наличия биопсии в анамнезе характеризуется точностью выявления РПЖ в среднем на уровне 49,9%, что выше средней точности выявления РПЖ при системной биопсии (около 40%). Таким образом, применение когнитивной МР-фьюжн-биопсии эффективно для пациентов вне зависимости от биопсии в анамнезе. Точность метода повышается при оценке МР-изображений с применением актуальной системы Pi-RADS v2, но на точность отрицательно может влиять локализация РПЖ в передней зоне.
Аппаратная МР-фьюжн-биопсия по сравнению с другими методами биопсии предстательной железы
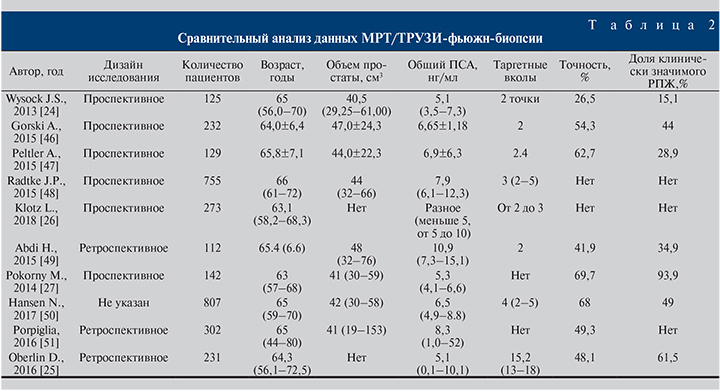
Все недостатки когнитивной МР-фьюжн-биопсии способна нивелировать аппаратная МР-фьюжн-биопсия. Суть метода в максимальной независимости от оператора путем использования оборудования, способного в режиме реального времени совмещать на одном экране в серой УЗ-шкале данные ТРУЗИ и МРТ. Главными недостатками метода считают несовпадение изображений при их сопоставлении во время подготовки к фьюжн-биопсии, и указанные цели не всегда содержат злокачественные клетки при выполнении фьюжн-биопсии. Однако, по данным [23], если придерживаться стратегии выполнения МР-таргетной биопсии на протяжении 5, 10, 15 и 20 лет, то на каждый период времени данная стратегия обойдется дешевле на 796, 956, 1616 и 2187 долл. соответственно, чем применяемая тактика обследования пациентов при помощи сБП [23]. Актуален вопрос сравнения эффективности аппаратной и когнитивной МР-фьюжн-биопсий предстательной железы. На этот вопрос попытались ответить J. S. Wysock et al., опубликовав данные исследования PROFUS [24]. По результатам обследования 125 пациентов общая точность диагностики РПЖ методами фьюжн-биопсии составила 32%, а точность аппаратной МР-фьюжн-биопсии по сравнению с когнитивной МР-фьюжн-биопсией составила 26,7 и 15,1% соответственно (р=0,1374, р=0,0523). D. Oberlin представили данные 231 пациента, из которых 81 была выполнена аппаратная МР-фьюжн-биопсия, 150 – когнитивная МР-фьюжн-биопсия предстательной железы. Когорты были стандартизованными по общему ПСА, Pi-RADS и семейному анамнезу. Общая выявляемость РПЖ оказалась выше в группе аппаратной МР-фьюжн-биопсии (48,1%) по сравнению с когнитивной (34,6%, р=0,4) и стандартной мультифокальной биопсией (32%, р=0,3) [25]. Важным фактором в пользу применения аппаратной МР-фьюжн-биопсии служит минимальное расхождение в определении стадии, дифференцировки РПЖ с результатами биопсии после операции. Показательным примером выявления опухоли более высокой степени злокачественности (upgrade), по данным таргетной аппаратной биопсии простаты, по сравнению с сБП стало исследование группы активного наблюдения L. Klotz et al., в котором у 273 пациентов в 33% случаев выявили повышение суммы баллов по Глисону [26]. M. Pokorny et al. в проспективном исследовании продемонстрировали феноменальную частоту выявления клинически значимого РПЖ (93,9%) при высокой точности диагностики РПЖ в целом (69,7%) в когорте пациентов без биопсии в анамнезе по результатам биопсии простаты при помощи аппаратного МР-фьюжн-наведения. По заключению авторов при использовании тактики наведения, основанной на МР-изображениях, диагностика РПЖ промежуточного/высокого риска увеличивается на 17,7% по сравнению с сБП [27]. Д. В. Долгачева и соавт. [28] продемонстрировали, что по результатам МР-фьюжн-биопсии при среднем возрасте пациентов 52 (43–74) года, уровне общего ПСА 4,2 (1,5–6,9) нг/мл у пациентов с биопсией в анамнезе (71,4% наблюдений) из 172 биоптатов, полученных при фьюжн-биопсии, РПЖ выявлен у 62 (36%). У 21 (50%) пациента РПЖ диагностирован только при фьюжн-биопсии, у 35,7% – только при сБП.
Фьюжн-биопсия простаты под контролем гистосканинга по сравнению с остальными таргетными методиками биопсии простаты
По результатам анализа данных исследования PICTURE L. Simmons et al. решили сравнить точность МР-фьюжн-биопсии и биопсии под контролем гистосканинга с промежностной биопсией предстательной железы у пациентов, нуждающихся в определении групп риска после трансректальной биопсии в анамнезе. Изначально целью исследования было определение отрицательной прогностической ценности МР-фьюжн-биопсии и биопсии под контролем гистосканирования. Были проанализированы данные 524 пациентов. Среди пациентов в 44% (230 больных) выявлен клинически значимый рак предстательной железы. При выявлении подозрительных на рак участков простаты для выполнения МР-фьюжн-биопсии (при балле по шкале Likert больше или равном 3) чувствительность, специфичность, положительное и отрицательное прогностические значения метода составили 97,14% (95% CI – 92–99), 21,9% (95% CI – 15,5–29,5), 46,7% (95% CI – 35,2–47,8) и 91,4% (95% CI – 76,9–98,1) соответственно. При выполнении фьюжн-биопсии под контролем гистосканирования, принимая объем подозрительного участка 0,5 мл и более в качестве значимого, чувствительность, специфичность, положительное и отрицательное прогностические значения метода составили 93,4% (95% CI – 86,2–97,5), 0,8% (95% CI – 0,0–4,2), 39,9% (95% CI – 33,3–46,8) и 91,4% (95% CI – 76,9–98,1) соответственно [29]. M. Hamann et al. исследовали выявляемость РПЖ путем применения технологии гистосканирования при промежностной биопсии предстательной железы.
В результате у 104 пациентов (средний возраст – 69 лет, средний уровень общего ПСА – 9,9 нг/мл) в 42% случаев диагностирован РПЖ. Частота выявления для каждой области была значительно выше при наведении с помощью гистосканирования по сравнению с сБП. Таким образом, использование системы гистосканирования для выявления области интереса перед биопсией простаты приводит к повышению частоты выявления РПЖ в данной группе пациентов [30]. В работе А. В. Говорова общая выявляемость РПЖ составила 35% (42/120). При сравнительной оценке методов авторами продемонстрировано преимущество сочетания МР-фьюжн-биопсии с сБП. Так, частота выявления РПЖ при выполнении стандартной трансректальной биопсии предстательной железы с дополнительными вколами под контролем гистосканирования увеличилась на 12% (73,8 против 85,7%; p<0,01), дополнительные вколы под контролем когнитивной МР-фьюжн-биопсии улучшили выявляемость рака при сБП на 19% (73,8 против 92,9%; p=0,04) [31]. Результаты исследования П. В. Глыбочко и соавт. также подтвердили возможность выявления РПЖ при помощи фьюжн-биопсии под контролем гистосканирования, посредством которого были определены участки, подозрительные на РПЖ, размером 0,5 см3 и более у 312 пациентов. Частота выявления РПЖ при сБП составила 59%, в то время как при выполнении биопсии под контролем гистосканирования – 87% [32]. Таким образом, гистосканирование при подозрении на РПЖ эффективно в сочетании со стандартной трансректальной биопсией предстательной железы. По данным А. В. Зубарева и соавт. [33], использование гистосканирования в диагностике РПЖ повышает чувствительность метода с 69,09 до 87,72%, выявляемость заболевания – с 67,24 до 75,86%.
На сегодняшний день визуализация РПЖ позволяет изменить подход к диагностике заболевания путем повышения эффективности биопсии – единственного на сегодняшний день метода верификации рака предстательной железы. «Золотым» стандартом биопсии в 2018 г. считают стандартную мультифокальную биопсию простаты. Однако из-за недостатков метода в диагностику РПЖ внедряют методы фьюжн-биопсии простаты под контролем МР- или УЗ-изображений, демонстрирующих свое превосходство. Выбор конкретной методики фьюжн-биопсии по-прежнему остается субъективным, большой ассортимент аппаратных решений может ввести специалистов в заблуждение. Помимо неоднозначности выбора конкретной методики при определенных исходных данных существуют сложности, возникающие во время самой процедуры фьюжн-биопсии: недостаточная точность при наличии подозрительных очагов в передней зоне простаты, использование всего одной проекции при таргетных вколах, смещение контура предстательной железы при движении ректального датчика, а также, по некоторым данным, риск увеличения частоты инфекционных осложнений. Рациональное применение антибактериальных средств в комбинации с фаготерапией позволит в значительной степени снизить число возникающих инфекционных осложнений. По причине того, что большинство исследований своей целью считают выяснение точности метода, его экономической эффективности, технические аспекты, как правило, не затрагиваются вообще и не учитываются при конечном анализе данных. Методики таргетной биопсии простаты с целью улучшения эффективности диагностики РПЖ по мере внедрения в практику врача-уролога РФ нуждаются в дальнейшем изучении.
В последние годы стандартная биопсия простаты под контролем УЗИ выполняется все реже, все чаще биопсию простаты выполняют с использованием какого-либо дополнительного метода визуализации. Эффективность методик МР-фьюжн-биопсии примерно одинаковая. Средняя выявляемость РПЖ при когнитивной и аппаратной МР-фьюжн-биопсиях составляет 49,9 и 52,6% соответственно.
В итоге можно сделать вывод, согласно которому оптимальная техника выполнения биопсии предстательной железы должна обладать самой высокой точностью обнаружения клинически значимого РПЖ при одновременно низкой частоте выявления клинически незначимого заболевания. Многими исследованиями показано, что выявляемость клинически значимого РПЖ при выполнении фьюжн-биопсии простаты выше, а клинически незначимого рака – ниже.
Использование гистосканирования в качестве инструмента для выполнения фьюжн-биопсии предстательной железы при выявлении подозрительных участков 0,5 см3 и более повышает эффективность выявления РПЖ.
Остаются неопределенными вопросы об оптимальном количестве вколов из подозрительных участков, о роли фьюжн-биопсии у первичных пациентов и у пациентов с биопсией в анамнезе. Одним из главных недостатков фьюжн-биопсии остается необходимость выполнения сБП. С целью улучшения эффективности фьюжн-биопсии предстательной железы планируется дальнейшее проведение исследований.



