Группа экспертов в урологии обсудили актуальную проблему диагностики и симптоматического лечения мужчин с доброкачественной гиперплазией предстательной железы (ДГПЖ) и гиперактивным мочевым пузырем (ГМП) на основании актуальных данных проведенных исследований и собственного клинического опыта.
Цель экспертного совета (ЭС) – актуализировать важность выявления сочетания симптомов нижних мочевыводящих путей (СНМП) у мужчин и выбора оптимальных схем терапии, что окажет влияние не только на выраженность данных симптомов, но и на основные показатели качества жизни.
Согласно рекомендациям Европейского общества урологов, СНМП принято делить на три основные группы: симптомы опорожнения, симптомы накопления и постмикционные. К симптомам опорожнения можно отнести затрудненное начало мочеиспускания, прерывистое мочеиспускание, слабую струю мочи, разбрызгивание струи мочи, необходимость в натуживании при мочеиспускании, капельное выделение мочи в конце мочеиспускания [1, 2, 6].
К симптомам наполнения можно отнести ургентность, недержание, учащенное мочеиспускание (поллакиурию), в том числе в ночное время (ноктурия).
Постмикционные симптомы – чувство неполного опорожнения мочевого пузыря и подкапывание мочи после мочеиспускания [1, 2].
Это условное деление СНМП помогает врачу при первичном осмотре выявить превалирующую симптоматику. СНМП разнообразны и неспецифичны, но их выявление важно для назначения симптоматической терапии и получения клинического ответа.
По данным эпидемиологических исследований, распространенность СНМП увеличивается с возрастом, а среди мужчин особенно увеличивается распространенность симптомов наполнения [3].
Средний возраст появления симптомов доброкачественной гиперплазии предстательной железы — 60 лет.
У 30% мужчин старше 65 лет обнаруживают развернутую клиническую картину заболевания. Однако увеличение простаты не всегда сочетается с клиническими проявлениями. Симптомы нижних мочевыводящих путей к 60 годам в какой-либо степени проявляются у 60% мужчин [5]. При обследовании мужчин европеоидной расы симптомы нижних мочевыводящих путей (от средних до значительных проявлений) были выявлены у 13% пациентов 40–49 лет, а у пациентов старше 70 лет — в 28% случаев [4, 5].
Современные исследования показывают, что беспокоящие СНМП могут иметь различные причины возникновения. К ним, в частности, относятся функциональные изменения мочевого пузыря. Доказано, что спонтанные сокращения детрузора приводят к возникновению частых, а иногда и ургентных позывов к мочеиспусканию. Такая совокупность симптомов расстройства фазы наполнения объединяется понятием «гиперактивный мочевой пузырь» (ГМП) [6].
Согласно определению Международного общества по проблемам, связанным с недержанием мочи (International Continence Society, ICS), синдром гиперактивного мочевого пузыря – это симптомокомплекс, сопровождающийся ургентностью и ноктурией, с недержанием мочи или без такового и учащенным мочеиспусканием в отсутствие доказанной инфекции мочевыводящих путей или другой очевидной патологии нижнего отдела мочевыводящих путей. Основным симптомом ГМП является неотложный (ургентный) позыв к мочеиспусканию, который обычно доминирует среди урологических симптомов мочевыводящих путей [6, 11].
В крупном международном популяционном исследовании EPIC показано, что среди мужчин симптомы наполнения преобладают над таковыми опорожнения (51,3, 25,7% соответственно) [4]. Однако, несмотря на широкое распространение данных симптомов, количество пациентов, получающих адекватную терапию, остается низким, так как они чаще получают терапию, направленную на лечение заболеваний предстательной железы, чем мочевого пузыря, возможно из-за привычки врачей связывать все СНМП у мужчин с заболеванием простаты [7].
Также следует принять во внимание высокую распространенность симптомов ГПМ у пациентов после ТУР. Согласно данным исследования А. А. Antunes, вероятность стойкой гиперактивности детрузора у некоторых групп пациентов составляла 83,3% [8].
С учетом многообразия симптомов врачу важно определить наиболее беспокоящие и учитывать необходимость персонифицированного подхода к терапии СНМП.
Согласно оценке эпидемиологии ГМП у мужчин старше 40 лет, самыми беспокоящими симптомами нижних мочевыводящих путей являлись ургентность, недержание мочи и ноктурия [9]. При этом симптомы ГМП у мужчин оказывают значительное влияние на ежедневную активность и качество жизни, включая работоспособность, социальные и семейные взаимоотношения и качество сна. Симптомы ГМП также негативно влияют на самочувствие, могут быть ассоциированы с тревогой и депрессией [9].
На сегодняшний день клинические руководства дают четкие рекомендации по терапии СНМП. Рассматриваемое состояние, безусловно, требует достаточно длительного лечения, и до начала медикаментозной терапии большинство специалистов предпочитают применять поведенческую терапию [6].
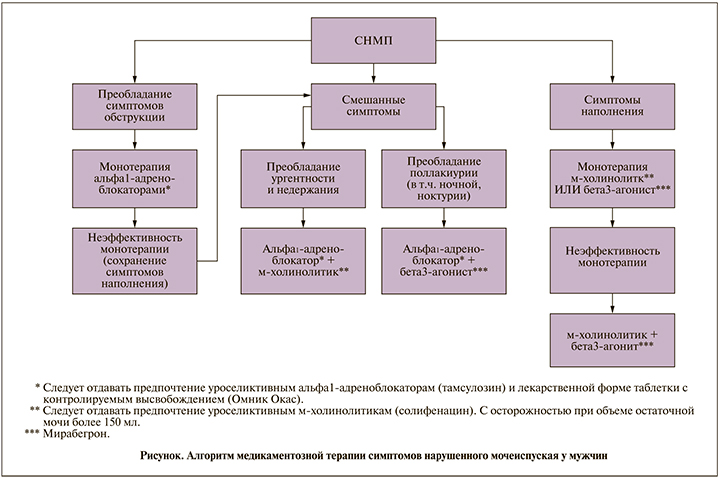
Согласно рекомендациям EAU, с учетом прогрессирующего характера ДГПЖ, медикаментозную терапию СНМП следует проводить длительно (иногда в течение всей жизни пациента). В зависимости от превалирования симптомов фазы опорожнения или накопления медикаментозная терапия может существенно различаться. Пациентам с симптомами ДГПЖ, с обструктивными симптомами в отсутствие задержки мочи лекарственную терапию рекомендуется начинать с уроселективных альфа1-адреноблокаторов. Международные данные свидетельствуют о высокой эффективности этих препаратов, заключающейся в 30–40%-ном уменьшении балла IPSS и увеличении максимальной скорости потока мочи приблизительно на 20–25% [6, 10].
При наличии симптомов наполнения, которые могут быть проявлением гиперактивности мочевого пузыря, рекомендуется назначать антагонисты мускариновых (антихолинергических) рецепторов, а также β3-агонисты адренорецепторов (β3-адреномиметики). Назначение антихолинергических ЛС позволяет снижать выраженность симптомов ГМП на 50–60% за счет нарушения передачи ацетилхолинового сигнала к гладкомышечным клеткам мочевого пузыря, так как они блокируют М2- и МЗ-холинорецепторы детрузора, за счет чего подавляется его патологическая сократительная способность в фазу наполнения, частота эпизодов ургентного недержания мочи снижается на 60–65%, частота мочеиспусканий – на 20–30% [6].
Комбинированное применение альфа1-адреноблокаторов и антагонистов мускариновых рецепторов рекомендовано пациентам с умеренными и тяжелыми СНМП при неэффективности предшествовавшей монотерапии одной из вышеуказанных групп препаратов. Больным, страдающим выраженной инфравезикальной обструкцией (Qmax ниже 5 мл/с), это лечение следует назначать с осторожностью, под контролем количества остаточной мочи (не более 150 мл). Высокая вероятность наличия ГМП у больных ДГПЖ обусловливает эффективность комбинированного применения альфа1-адреноблокатора и средств с антимускариновой активностью. Первый класс препаратов эффективно устраняет динамический компонент (функциональный), симптомы инфравезикальной обструкции, в то время как второй способен значительно снижать выраженность накопительных расстройств [6].
Некоторые пациенты резистентны к М-холинолитикам [12]. Кроме того, применение данных средств не сопровождается достаточной комплаентностью со стороны больных [13]. Принимая во внимание проблемы, связанные с лечением СНМП, встает вопрос о необходимости применения и других фармакологических препаратов или иных методов коррекции симптомокомплекса ГМП. Мирабегрон – мощный селективный агонист бета3-адренорецепторов. В экспериментальных исследованиях мирабегрона продемонстрировано расслабление гладких мышц мочевого пузыря у крыс и в изолированном препарате человеческих тканей, а также увеличение концентраций цАМФ в тканях мочевого пузыря у крыс. Таким образом мирабегрон улучшает резервуарную функцию мочевого пузыря за счет стимуляции бета3-адренорецепторов, расположенных в его стенке [14].
В рандомизированных плацебо-контролируемых клинических исследованиях продемонстрирована эффективность мирабегрона как для пациентов, ранее получавших M-холиноблокаторы в качестве лечения гиперактивного мочевого пузыря, так и для пациентов без анамнеза предыдущей терапии последними. Мирабегрон также был эффективен для пациентов с ГМП, которые прекратили лечение M-холиноблокаторами из-за отсутствия эффекта [15]. Таким образом, применение мирабегрона мужчинами с СНМП и ГМП как в монотерапии, так и в комбинации с альфа-блокаторами может быть дополнительной опцией для терапии данной категории пациентов.
Несмотря на существующие исследования, на сегодняшний день нет четких рекомендаций по применению комбинации альфа1-адреноблокаторов и β3-агонистов адренорецепторов. В исследовании MATCH study-комбинация тамсулозин+мирабегрон продемонстрировала лучшую эффективность, чем тамсулозин+плацебо для 565 мужчин с ГМП+ДГПЖ [16]. В другом исследовании комбинация тамсулозина и мирабегрона также была более эффективной, чем тамсулозин и плацебо для 94 пациентов с ГМП и ДГПЖ [17].
В сводном анализе пяти исследований III фазы (Scorpio/Aries/Capricorn/Beyond/Taurus) показано, что мирабегрон у мужчин достоверно снижает число мочеиспусканий в сутки, но не было выявлено статистической разницы между мирабегроном и плацебо по симптомам ургентности и ургентного недержания мочи [18]. Долгосрочные исследования Везикара, наоборот, демонстрируют его хороший эффект относительно симптомов ургентности и ургентного недержания мочи. Так, в исследовании Haab прием Везикара снижал частоту эпизодов ургентности на 89% к 52-й неделе лечения, 60% пациентов с недержанием мочи избавились от этого симптома [19]. При недостаточной эффективности М-холинолитиков или β3-агонистов возможна их комбинация, что подтверждено данными нескольких исследований, в том числе долгосрочных [20–22].
ВЫВОДЫ
- Эпидемиологические исследования подтверждают высокую распространенность сочетания симптомов опорожнения и наполнения у мужчин, что может потребовать дополнительного назначения к альфа1-бета-блокаторам м-холинолитиков и бета3-агонистов.
- Пациентам с изолированными обструктивными симптомами (затрудненное начало мочеиспускания, прерывистое мочеиспускание, слабая струя мочи, разбрызгивание струи мочи, необходимость в натуживании при мочеиспускании, капельное выделение мочи в конце мочеиспускания) рекомендовано назначение уроселективных альфа1-адреноблокаторов. Для снижения числа побочных явлений следует отдавать предпочтение таблеткам с контролируемым высвобождением препарата.
- При наличии у пациента смешанных симптомов с преобладанием ургентности и/или ургентного недержания мочи рекомендуется назначать комбинированную терапию альфа1-адреноблокаторами и М-холинолитиками.
- При наличии у пациента смешанных симптомов с преобладанием поллакиурии и/или ноктурии рекомендуется назначать комбинированную терапию альфа1-адреноблокаторами и β3-агонистами адренорецепторов.
- Пациентам с СНМП и преобладанием симптомов наполнения при неэффективности монотерапии рекомендовано назначение комбинированной терапии М-холинолитиками и β3-агонистами.
По результатам работы экспертного совета предложен и может применяться в клинической практике следующий алгоритм (см. рисунок).