Введение. Доброкачественная гиперплазия предстательной железы (ДГПЖ) – связанное с возрастом прогрессирующее заболевание, сопровождающееся постепенным увеличением ее объема, симптомами нижних мочевыводящих путей (СНМП), снижением скорости мочеиспускания, осложняющегося эпизодами острой задержки мочи (ОЗМ), ухудшением качества жизни, присоединением опасных осложнений и нередко потребностью в хирургическом вмешательстве. Увеличение продолжительности жизни и числа мужчин старшего и преклонного возраста, улучшение качества медицинской помощи и социально-экономических показателей в большинстве стран способствовали росту числа больных ДГПЖ [1].
Первоочередная задача лечения при ДГПЖ заключается в быстром и эффективном контроле над СНМП в течение длительного периода. До недавнего времени основным методом лечения больных ДГПЖ был оперативный. Однако достижения фундаментальной науки в изучении основных механизмов патогенеза, а также успехи фармации в разработке современных лекарств настолько значимы, что медикаментозная терапия стала первой линией лечения [2]. На этой основе разработана новая стратегия терапии больных ДГПЖ, в основе которой лежит улучшение и сохранение качества пациента, предупреждение осложнений и необходимости оперативного вмешательства.
В патогенезе ДГПЖ наряду с другими механизмами установлено повышение активности 3α-оксиредуктазы, что приводит к снижению концентрации 5α-андростандиола, способствующему повышению количества и активности α1-АР [3]. В ткани ДГПЖ подтип α1А-АР встречается чаще всего. Стимуляция α1А-АР при ДГПЖ клинически проявляется ирритативной симптоматикой и ухудшением качества жизни пациента. Повышенная стимуляция α1-АР приводит к увеличению тонуса гладкой мускулатуры шейки мочевого пузыря, простатического отдела уретры, ПЖ и поддерживает повышенное внутриуретральное давление. Поскольку α1-АР в основном локализуются в стромальном компоненте ПЖ, занимающем 60% ее объема, увеличение их количества и их активация обусловливают динамический компонент инфравезикальной обструкции. По мере прогрессирования заболевания развиваются функциональные и морфологические изменения детрузора с нарушением кровообращения и гипоксии тканей.
Стратегия лечения ДГПЖ эволюционировала от преимущественно оперативной к консервативной. Задачи медикаментозного лечения больных ДГПЖ: краткосрочные – подготовка больного к оперативному лечению; долгосрочные – сохранить и улучшить качество жизни пациента, предотвратить осложнения, связанные с инфравезикальной обструкцией, свести к минимуму потребность в оперативном вмешательстве, что особенно важно в случаях тяжелых сопутствующих заболеваний. Определены основные принципы консервативного лечения больных ДГПЖ: лечить больного ДГПЖ, а не ДГПЖ у больного; приступать к лечению только после исключения рака ПЖ и оценки активности и характера сопутствующего воспалительного процесса в ПЖ; полная информированность пациента о существующих методах лечения и их возможных осложнениях; использование для лечения лекарственных средств, эффективность которых основана на принципах доказательной медицины; диспансерное наблюдение за пациентом; использование объективных критериев оценки эффективности проводимого лечения и их обсуждение с пациентом [4].
В настоящее время α1-адреноблокаторы являются первой линией лечения СНМП при ДГПЖ [5]. Все современные препараты этой группы нашли широкое применение в клинической практике, получили должную оценку и по существу отличаются только выраженностью возможных нежелательных реакций (НР). Она зависит не только от степени их селективности к различным подтипам рецепторов, но и от их тропности к тканям. Сравнение специфичности α1-адреноблокаторов к различным подтипам α1-АР и их возможные НР представлены в табл. 1 [6–10].

Установлено, что урологи (23%) и врачи общей практики (34%) переоценивают распространенность сексуальной дисфункции, обусловленной препаратами, используемыми при лечении ДГПЖ, хотя известно, что их частота не превышает 10%. По данным зарубежных исследований, частота ретроградной эякуляции при терапии препаратами этой группы достигает 28,1% для силодозина, 11% для тамсулозина и 1% для алфузозина [11]. В то же время расстройства в сексуальной сфере пациенты воспринимают как не менее существенные по сравнению с другими НР, которые могут возникать на фоне приема препаратов для лечения ДГПЖ. У 17,4% больных, принимающих тамсулозин, и 14,2% – силодозин, развитие расстройств в сексуальной сфере, по мнению респондентов, может стать даже причиной отказа от дальнейшего лечения [11]. У других представителей группы α1-адреноблокаторов этот показатель находится в пределах 7–8% [11]. Тем не менее у многих пациентов с ДГПЖ возникает необходимость в коррекции ЭД при проведении консервативного лечения. По данным Mondul et al. [12], при наличии у мужчин СНМП риск развития ЭД оказался выше на 40%. Международные исследования показали, что многие больные, имеющие СНМП вследствие ДГПЖ, считают для себя важным сохранение половой активности [12].
За последние годы появились данные, указывающие на довольно сильную взаимную связь ЭД и СНМП. Известно, что повышенная экспрессия фермента фосфодиэстеразы (ФДЭ) 5-uj-типа в тканях мочевого пузыря, полового члена, ПЖ, задней уретры, в т.ч. и у больных ДГПЖ с ЭД, препятствует оксид-азота (NO)-зависимому расслаблению гладкой мускулатуры, что способствует ее повышенному тонусу, повышенной пролиферации в тканях этих органов, усугублению нарушений кровообращения. По последним данным, наиболее вероятными считаются четыре механизма взаимосвязи между ЭД с СНМП: уменьшение содержания NO, нарушение регуляции Rho-киназы, гиперактивность вегетативной нервной системы и атеросклероз сосудов малого таза [13]. Эти звенья патогенеза ЭД и СНМП могут как действовать раздельно, так и дополнять друг друга. Препараты группы ингибиторов ФДЭ-5 способны повышать содержание NO в тканях, что приводит к расслаблению гладкой мускулатуры, увеличению притока крови в область малого таза, половому члену, сокращению гладкой мускулатуры, улучшению половой функции и регрессу СНМП [4].
Эректильная дисфункция и эякуляторные нарушения тесно связаны с возрастом и тяжестью СНМП [14]. По данным научной литературы, с возрастом частота ЭД прогрессивно растет и к 70 годам составляет до 64%. Распространенность СНМП также увеличивается с возрастом. Так, в исследовании МSAM-7 среди 12 тыс. мужчин в возрасте 50–80 лет распространенность СНМП, обусловленных ДГПЖ, составила 90%. ЭД была выявлена у 49% мужчин, из них полное отсутствие эрекции было у 10%.
У 66% мужчин наряду с СНМП присутствовали нарушения эрекции. В целом нарушения эякуляции выявлены у 46% пациентов, из них у 61% с СНМП.
Таким образом, имеющаяся патогенетическая взаимосвязь между ДГПЖ и ЭД требует разработки комплексного подхода к лечению этих нозологий. В исследованиях [15, 16] представлены результаты сочетанного использования α1-адреноблокаторов и ингибиторов ФДЭ-5 при лечении СНМП, которые указывают на эффективность такой комбинированной терапии больных с сочетанием ЭД и ДГПЖ. В связи с этим помимо уточнения эффективности этой терапии особую актуальность приобретает оценка влияния такого лечения на качество жизни пациентов, на частоту и тяжесть побочных эффектов.
Цель исследования: оценить эффективность и безопасность применения комбинированной фармакотерапии α1-адреноблокатором и ингибитором ФДЭ-5 для пациентов с ДГПЖ и ЭД.
Материалы и методы. В наблюдательную мультицентровую программу с участием 18 лечебно-профилактических учреждений Москвы вошли 315 мужчин в возрасте 40–65 лет (средний возраст – 55,2 года) с установленным диагнозом ДГПЖ и ЭД.
Критерии включения в исследование: мужчины в возрасте 40–65 лет включительно, которым по показаниям в соответствии с инструкцией по применению препарата назначено комбинированное лечение Алфупрост® МР (алфузозин) и Виатайл® (силденафил); наличие расстройства мочеиспускания, соответствующее 8 баллам и более по шкале IPSS, в сочетании сЭД (≤ 20 баллов по шкале МИЭФ-5); приверженность назначенному в рамках исследования медикаментозному лечению в течение 3 мес. и более; подписанное информированное согласие пациента и согласие на обработку персональных данных.
Критерии невключения: прием других препаратов группы α1-адреноблокаторов или ингибиторов ФДЭ-5 в течение последнего месяца; состояние, препятствующее применению алфузозина или силденафила в соответствии с противопоказаниями, мерами предосторожности и специальными предупреждениями, указанными в Инструкции по медицинскому применению препаратов Алфупрост® МР и Виатайл®; известные аллергические реакции на алфузозин или силденафил; психические заболевания; наличие в анамнезе хирургического лечения по поводу ДГПЖ; наличие абсолютных показаний к хирургическому лечению ДГПЖ; объем остаточной мочи более 150 мл; морфологически подтвержденный рак предстательной железы; воспалительные заболевания органов мочеполовой системы в стадии обострения; наличие в анамнезе оперативных вмешательств по поводу стриктуры уретры и заболеваний мочевого пузыря; неврологические расстройства, влияющие на функции накопления и опорожнения мочи; сопутствующие заболевания в стадии суб- и декомпенсации.
Исследование предусматривало 3 визита: исходный (визит 1), через 1,5 и 3 мес. после начала лечения – визиты 2 и 3 соответственно. При обследовании фиксировали жалобы, анамнез, результаты физикального осмотра, данные опросников IPSS, QоL и МИЭФ-5, определяли уровень простатического специфического антигена (ПСА) крови, проводили трансректальное УЗИ простаты для определения ее объема, УЗИ мочевого пузыря для определения объема остаточной мочи, а также урофлоуметрию для уточнения максимальной объемной скорости потока мочи (Qmax). В ходе динамического наблюдения оценивали изменение качества жизни (регресс СНМП и улучшение сексуальной функции), а также фиксировали НР.
Все пациенты получали комбинированную терапию, включившую Алфупрост® МР (алфузозин) 10 мг/сут и Виатайл® (силденафил) 50 мг/сут (при необходимости дозу увеличивали до 100 мг/сут). В увеличении дозы силденафила до 100 мг/сут в связи с недостаточной эрекцией нуждались 20 (6,35%) больных.
Сбор данных осуществляли с помощью стандартных опросников, заполненных пациентами рутинно на визитах, и специально разработанной анкеты, которую заполнял лечащий врач. Все данные, полученные в ходе исследования, заносили в базу данных для последующей статистической обработки. Математическая и статистическая обработка полученных данных проведена с использованием стандартных пакетов программ Statistica (V7.0) и SPSS Statistics (V17.0). Для обработки данных были использованы методы описательной статистики.
Результаты и обсуждение. По результатам обследования ДГПЖ и ЭД сопутствовали ожирение (27,3%), курение (58,4%), употребление алкоголя (67,7%), андропауза (22,9%), артериальная гипертония (52,5%), сахарный диабет (13,6%), снижение физической активности (43,3%). Средний индекс массы тела составил 27,2 (21–38) кг/м2, уровень общего ПСА – 2,5 (2–4) нг/мл.
На визите 1 пациенты чаще всего предъявляли жалобы на вялую струю мочи (85%), учащенное мочеиспускание (79%), затрудненное мочеиспускание (85,9%) и ноктурию (68,2%; табл. 2). Исходный балл IPSS в среднем составил 12,1, QoL – 2,81. Увеличение предстательной железы, по данным пальцевого ректального исследования, констатировали у 284 (98,07%) пациентов, болезненность – у 76 (25%), узловые образования – у 37 (12,0%).
В результате проведенного лечения удалось добиться регресса СНМП. Характерным оказалось исчезновение боли, на 62% уменьшилась частота поллакиурии, на 58% – частота ноктурии, на 64% – частота затруднений при мочеиспускании. Средний объем остаточной мочи после лечения уменьшился на 38,7%, средний показатель Qmax вырос до нормальных значений – 17,42±2,99 мл/с, что определило эффективный спазмолитический эффект лечения (см. табл. 2). Снижение индекса IPSS c 12,1 до 8,2 балла и повышение индекса QoL с 2,8 до 1,8 балла были статистически значимыми (р<0,05) и подтверждали эффективность проводимого лечения.
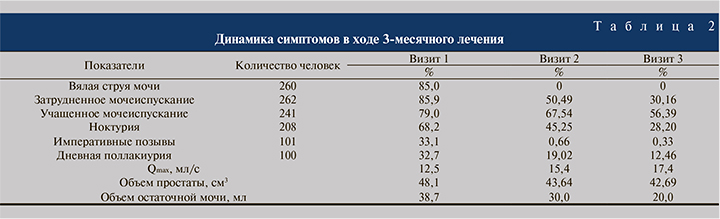
Необходимо подчеркнуть, что главным преимуществом α1-адреноблокаторов в терапии СНМП, обусловленных ДГПЖ, является быстрый клинический эффект (в первые 3–5 сут.). Это подтверждают результаты проведенного исследования: выраженность симптомов значимо снизилась уже через 1,5 месяца терапии (к визиту 2), эта тенденция сохранялась и к визиту 3. Клиническое улучшение не сопровождалось значительным ростом показателя Qmax – не более 5 мл/с у большинства больных к визиту 3. Эти данные, свидетельствующие против ведущей роли механического фактора инфравезикальной обструкции, подтверждены незначительным изменением объема увеличенной простаты по данным трансректального УЗИ, а также сохранением объема остаточной мочи менее 100 мл, который после лечения уменьшился на 38,7%. Следует учитывать, что СНМП у пациентов, включенных в исследование, могли быть также обусловлены метаболическими нарушениями на фоне сопутствующей соматической патологии (ишемическая болезнь сердца, артериальная гипертензия, сахарный диабет, ожирение).
Особенно ценным было изменение оценки сексуального здоровья мужчин, поскольку оно в большей степени определяет качество жизни, нежели симптомы нарушения мочеиспускания (табл. 3). Исходно средние показатели по всем вопросам шкалы МИЭФ-5 свидетельствовали о тяжелой и среднетяжелой ЭД. Для большей объективности и уточнения характера половой дисфункции каждый вопрос был оценен индивидуально. Так, на вопрос: как Вы оцениваете степень Вашей уверенности в том, что Вы можете достичь и удержать эрекцию, 6,5% пациентов показали очень низкую степень уверенности, 89,8% – низкую и среднюю и только 3,6% пациентов – высокую степень уверенности.

На визите 3 самый низкий балл (3,72) получил вопрос об оценке уверенности возможности достичь и удержать эрекцию. И хотя это значение оказалось существенно выше исходного (2,54), страх перед половой связью сохранялся и, вероятно, требуется время для закрепления положительного эффекта лечения. Не исключено, что на этот субъективный фактор влияют длительность половой дисфункции и уровень тестостерона в крови. Среди баллов преобладает достаточная степень уверенности в достижении и удержании эрекции у 58,3%. Значительно лучше были показатели степени достаточности эрекции для введения полового члена во влагалище. У 80,64% больных эрекция была высокой и очень высокой степени. Следует отметить, что эффект уверенности был достигнут на 2-м месяце лечения и далее оставался стабильным. Достаточность эрекции перед введением полового члена во влагалище отметили 246 (80,6%) больных, что расценивается как высокая и очень высокая степень восстановления эрекции. Сохранение достаточной эрекции после введения полового члена во влагалище по окончании лечения наблюдали у 84,3% пациентов. Удовлетворенность половым актом высокой и очень высокой степени отметили 84,6%.
Данные литературы показывают, что положительное влияние α1-адреноблокаторов на функцию нижних мочевых путей и мужских половых органов опосредовано несколькими механизмами:
- местное воздействие на адренорецепторы шейки мочевого пузыря, простаты и проксимального отдела уретры;
- ликвидация ишемии органов малого таза вследствие улучшения притока артериальной крови, повышения сократительной способности детрузора;
- нормализация кровообращения спинальных центров и проводящих путей вегетативной нервной системы, обеспечивающих функцию нижних мочевыводящих путей через центральные воздействия на подкорковые регуляторы этой функции [17];
- влияние на апоптоз и липидный обмен детрузора;
- улучшение эрекции.
α1D-рецепторы являются ведущими в расслаблении мышечной стенки мочевого пузыря [9]. Для облегчения как обструктивных, так и ирритативных симптомов важно блокировать α1А- и α1D- АР [18]. Блокируя эти подтипы АР, алфузозин влияет как на обструктивные (опорожнение), так и на ирритативные (накопление) симптомы. Алфузозин обладает самым высоким баллом простатселективности среди других представителей группы α1-адреноблокаторов (отношение концентрации препарата, необходимого для блокады 50% АР почечной артерии и ткани простаты) [19, 20].
За весь период наблюдения различные НР на фоне комбинированной терапии диагностированы в 2,95% наблюдений (n=9) на визите 2, у 5 (1,64%) пациентов зарегистрирована РЭ. Также зафиксированы НР, характерные для приема силденафила (покраснение лица, заложенность носа, головная боль, зрительный дискомфорт). На визите 3 наиболее часто регистрировали заложенность носа (1,31%) и головную боль (0,98%), частота выявления РЭ составила 0,66%, еще реже (0,33%) отмечали головокружение. Тщательный анализ НР выявил от 0,9 до 0,3% НР, характерных для α1-адреноблокаторов, что существенно ниже, чем при использовании других представителей этой группы препаратов. Поскольку препараты группы α1-адреноблокаторов могут вызывать ортостатического гипотензию, АД определяли на каждом визите. Средние показатели АД на визите 1 составили 130,60/81,61 мм рт.ст. и понизились на визите 3 до средних значений 128,53/79,32 мм рт.ст. Таким образом, на фоне применения Алфупроста® МР было отмечено клинически незначимое снижение АД, которое не влияло на самочувствие пациентов и не ухудшало качества их жизни. Низкая частота развития НР на фоне приема Алфупроста® МР во многом связана с использованием запатентованной технологии производства таблеток матричного типа, благодаря которой стало возможно медленное (модифицированное) высвобождение действующего вещества (алфузозина) и позволило контролировать его концентрацию в плазме, предотвращая появление пиковых концентраций. Технология модифицированного высвобождения делает возможным однократный прием Алфупроста® МР вне зависимости от времени суток и позволяет назначать препарат коморбидным пациентам.
Удовлетворенность пациента и врача проведенным лечением оценивали с помощью опросника удовлетворенности Лайкерта (1 балл – полностью не удовлетворен, 2 балла – не удовлетворен; 3 балла – удовлетворен и не удовлетворен; 4 балла – удовлетворен; 5 баллов – полностью удовлетворен). В целом средний показатель наших пациентов составил 4,2, оценка эффективности как высокая и очень высокая (по градации опросника Лайкерта) составила 91,1%. Средний показатель оценки врачом удовлетворенности клиническим ответом пациентов на проведенную терапию по завершении лечения, полученный с использованием той же шкалы, составил 4,35 балла, что соответствует высокой и очень высокой эффективности.
Таким образом, применение комбинированной терапии препаратами Алфупрост® МР 10 мг и Виатайл® 50 мг (при необходимости 100 мг) в практике лечения ДГПЖ и ЭД показало высокий клинический эффект и благоприятный профиль безопасности: симптомы нарушенного регрессировали более чем на 60%, качество жизни выросло на 64%, эректильная функция улучшилась более чем у 80% больных; НР имели место у 0,9% больных.
Заключение. В настоящее время имеются ограниченные данные в пользу рекомендаций в рутинной практике сочетания α1-адреноблокаторов с ингибиторами ФДЭ-5 при лечении ДГПЖ и ЭД. Наши результаты продемонстрировали высокую эффективность и благоприятный профиль безопасности применения комбинированной терапии Алфупростом® МР (алфузозин) и Виатайлом® (силденафил) пациентов с СНМП и ЭД, что в отсутствие риска прогрессирования заболевания позволяет рассматривать ее как оптимальный вариант лечения.



