Введение. Диагноз мужского бесплодия касается не только личной жизни, но и социальной и экономической сфер общества. Согласно последним данным, мужское бесплодие наравне с женским приводит к вынужденной бездетности супругов и демографическим проблемам [1]. Портфолио фармацевтических компаний не пополняется одобренными препаратами для лечения мужского бесплодия, тем не менее на сегодняшний день обнаружено 74 активных клинических испытания (clinicaltrials.gov), посвященных данному диагнозу, что подтверждает повышенное внимание к этой теме и неудовлетворенные потребности пациентов.
Большинство клинических исследований сосредоточено на антиоксидантных соединениях [2]. Окислительный стресс в сперме является одним из ключевых патогенетических механизмов, лежащих в основе идиопатического мужского бесплодия. Обнаружено большое число пусковых механизмов развития бесплодия, и многие из них приводят к дисбалансу активных форм кислорода (АФК), что в свою очередь приводит к нарушениям и изменениям сперматогенеза. В частности, окислительный стресс приводит к повреждению ДНК, усилению перекисного окисления липидов мембран, нарушению регуляции антиоксидантной системы (например, активности супероксиддисмутазы [СОД]). Все упомянутые выше процессы с возрастом ускоряются [3].
Мужское бесплодие сильно коррелирует с возрастом [4], поэтому модели репродуктивного старения являются хорошим объектом для изучения терапевтического воздействия при мужской инфертильности. В частности, в данном исследовании использовалась модель старения с помощью D-галактозы [5]. У мышей, которым вводили D-галактозу, наблюдалось снижение активности СОД и повышенное перекисное окисление липидов [6]. Низкая концентрация СОД в свою очередь влияет на экспрессию специфических генов, ответственных за процесс сперматогенеза, приводит к снижению общего числа сперматозоидов и, в частности, к увеличению числа неподвижных и аномальных сперматозоидов.
Несмотря на то что на сегодняшний день уже появились данные о роли регуляторных пептидов в процессах сперматогенеза, терапевтический потенциал пептидов при мужском бесплодии все еще остается малоизученным [7]. Сперматогенез является сложноорганизованным процессом, который проходит через такие стадии, как митоз, мейоз, клеточное дифференцирование, и требует четкой координации всех звеньев механизма. Данную роль выполняют эндогенные регуляторные пептиды, локально высвобождаемые в семенниках в результате протеолиза и формирующие пул для поддержания последовательного процесса сперматогенеза. В недавних исследованиях описана важная роль регуляторных пептидов, полученных клетками Сертоли из некоторых структурных белков мембраны семенного канальца (например, ингибина, ламинина, коллагена), в процессах клеточной дифференцировки от сперматогоний к сперматозоидам и движения зародышевых клеток вдоль клеток Сертоли, а также участие пептидов в перекрестных взаимодействиях между базальной мембраной и семенным эпителием [9] и в модуляции гемато-тестикулярного барьера (ГТБ) [10] – весь пептидный пул в совокупности создает правильные условия для полноценного процесса сперматогенеза.
Не следует забывать об антиоксидантных свойствах регуляторных пептидов семенников. Пептиды не только повышают устойчивость клеток к окислительному стрессу [11], но и ускоряют восстановление после АФК-индуцированного повреждения [12]. Антиоксидантная терапия рекомендована для медикаментозного лечения мужского бесплодия, поэтому данный механизм действия лекарственных препаратов на основе регуляторных пептидов семенников может иметь решающее значение для восстановления мужской фертильности [13].
Препарат Фертивелл, пептидный комплекс на основе полипептидов семенников крупного рогатого скота, в двойных слепых плацебо-контролируемых рандомизированных клинических исследованиях продемонстрировал клинический потенциал для лечения мужского бесплодия за счет повышения количества сперматозоидов, улучшения их подвижности и восстановления количества морфологически нормальных форм у мужчин с нарушениями сперматогенеза [14]. Целью данного исследования является изучение специфических механизмов действия препарата Фертивелл на мышиной модели репродуктивного старения, вызванного D-галактозой.
В качестве стандарта лечения была выбрана комбинация L-карнитина и ацетил-L-карнитина. Данные соединения являются метаболическим средством в комплексной терапии мужского бесплодия [13]. Высокая концентрация L-карнитина и ацетил-L-карнитина наблюдается в придатках семенников и играет решающую роль в метаболизме и созревании сперматозоидов – комплекс влияет на подвижность сперматозоидов и проявляет выраженный антиоксидантный эффект [15]. Таким образом, сравнение с L-карнитином и ацетил-L-карнитином дает возможность оценить антиоксидантные свойства и выявить другие эффекты препарата Фертивелл.
Материалы и методы
Реагенты
В исследовании были использованы реактивы: LIVE/ DEAD Viability Kit, Mitotracker Green, CellRox Deep Red (Invitrogen), Sigma ДМСО, L-карнитин, ацетил-L-карнитина гидрохлорид (Abcam), D-галактоза (Sigma).
Дизайн исследования
Самцы мышей линии C57BL/6J в возрасте 5–6 нед. были получены из питомника «Пущино» Института биоорганической химии им. Шемякина–Овчинникова РАН. Все эксперименты на животных проводились в соответствии с европейскими и российскими национальными руководствами по проведению экспериментов на животных и были одобрены Локальным комитетом по биоэтике ФГБУ «НМИЦ онкологии им. Н. Н. Блохина», регистрационный номер 04P–072021.
После акклиматизации в течение 1 нед. мышей линии C57BL/6J рандомизировали на четыре группы (по 20 мышей в каждой, рис. 1): интактная группа (Ctrl), контрольная группа, получавшая D-галактозу (Gal); группа, получавшая D-галактозу и Фертивелл (PP); группа, получавшая D-галактозу и комбинацию L-карнитина и ацетил-L-карнитина (LC). Репродуктивное старение вызывали в трех группах внутрибрюшинным (в/б) введением D-галактозы ежедневно в дозе 100 мг/кг в течение 8 нед. [16]. Группа интактных мышей (Ctrl) получала физиологический раствор внутрибрюшинно по 0,2 мл ежедневно на протяжении всего эксперимента. После завершения введения D-галактозы (день 56) мышам ежедневно внутрибрюшинно вводили либо Фертивелл в дозе 1 мг/кг (PP), либо смесь L-карнитина 400 мг/кг с ацетил-L-карнитином 200 мг/кг (LC), либо физиологический раствор (Gal) в течение 5 нед. По завершении введения соединений мышей подвергали эвтаназии через 1, 10, 20, 30 дней после окончания терапии (временные точки – TP) и получали образцы спермы для оценки подвижности и качества, а образцы семенников готовили для определения мРНК и иммуногистохимического (ИГХ) анализа.
Параметры спермы
Сперматозоиды собирали из семявыносящего протока мышей и суспендировали в среде Ham’s F10 (Invitrogen). Для определения процента подвижности среду для сперматозоидов разбавляли до 106/мл средой Ham’s F10 и подсчитывали в камере Маклера с сеткой для подсчета сперматозоидов (Sefi Medical). Для определения подвижности сперматозоидов в двух повторах анализировали не менее 200 сперматозоидов (как подвижных, так и неподвижных). Для анализа митохондриальной активности образцы сперматозоидов окрашивали Mitotracker Green (10 мг/мл) (Invitrogen, Карлсбад, Калифорния, США); CellRox DeepRed (Invitrogen, Карлсбад, Калифорния, США), DAPI (Invitrogen, Карлсбад, Калифорния, США) использовали для окрашивания ядер. Анализ окрашивания проводили с помощью проточной цитометрии (Novocyte 2000R, Agilent, США) и конфокальной микроскопии (INCell Analyzer 6000, GE Healthcare).
Анализ содержания тестостерона в образцах
Образцы крови мышей собирали в пробирки с активатором свертывания для получения сыворотки BD Vacutainer® Plus Serum (BD, США) на 1-е, 10-е, 20-е, 30-е сутки (см. раздел 2.2. Дизайн эксперимента). Полученную кровь в пробирках инкубировали при комнатной температуре 30 мин, затем центрифугировали 10 мин при 3000 об/мин. Сразу после центрифугирования сыворотку отделяли от сгустка и осветляли при 10000 g на центрифуге (Eppendorf, Германия). В лунки 96-луночного планшета в двух повторах вносили по 25 мкл калибровочных образцов и контрольной сыворотки. Остальные лунки заполняли в двух повторах по 25 мкл исследуемых образцов сыворотки. Во все лунки добавляли по 100 мкл конъюгата. Планшет запечатывали пленкой для герметизации планшета и инкубировали в течение 60 мин при +37°С и постоянном встряхивании на шейкере-инкубаторе при 600 об/мин. По окончании инкубации содержимое лунок удаляли и лунки 5 раз промывали 250 мкл промывочного раствора. Затем во все лунки вносили по 100 мкл раствора тетраметилбензидинового субстрата и инкубировали планшет в темноте при комнатной температуре (+18...+25°С) в течение 20 мин. Стоп-реагент по 100 мкл добавляли во все лунки с той же скоростью и в той же последовательности, что и раствор тетраметилбензидинового субстрата, при этом содержимое окрашивалось в ярко-желтый цвет. Оптическую плотность лунок планшета измеряли на фотометре Multiscan FC при 450 нм (Thermo Scientific, Китай).
Выделение мРНК и ПЦР в реальном времени
По экспрессии мРНК можно оценить присутствие белка (или его отсутствие), участвующего на том или ином этапе сперматогенеза. На основании поиска литературных данных были отобраны гены, белки которых принимают участие в ключевых процессах развития и созревания сперматозоидов. Был произведен подбор праймеров и определена экспрессия мРНК методом ОТ-ПЦР (полимеразная цепная реакция с обратной транскрипцией) для определения активности генов. РНК выделяли с помощью мини-набора RNeasy plus (Qiagen), позволяющего отделить геномную ДНК с помощью разделительных колонок. Гомогенизацию тканей проводили с использованием системы TissueLyser LT (Qiagen). Образцы РНК хранили при температуре -80°C; эксперимент был смоделирован таким образом, чтобы исключить несколько циклов замораживания-оттаивания материала при работе.
Дизайн праймеров был выполнен с использованием онлайн-сервисов и баз данных. Последовательности мРНК для дизайна праймеров были выбраны с использованием базы данных геномного браузера Ensembl. Затем нуклеотиды, расположенные на границе экзона, были вручную сконструированы (по возможности для мишеней), чтобы минимизировать риски обнаружения сигнала от остаточных количеств геномной ДНК. Формирование вторичных структур проверяли с помощью сервиса OligoAnalyzer Tool; специфичность систем праймеров определяли с помощью сервиса PrimerBlast.
Стадирование ПЦР в реальном времени проводили с использованием набора OneTube RT-PCR SYBR (Евроген, Россия), позволяющего проводить реакцию обратной транскрипции и амплификацию в одной пробирке (что повышает чувствительность реакции). Каждый анализ включал выполнение контроля обратной транскрипции и отрицательного контроля реагентов в дополнение к целевым образцам в двух повторах.
Иммуногистохимическое (ИГХ) окрашивание образцов семенников
Определение экспрессии маркеров приводили при помощи ИГХ-окрашивания на образцах семенников мышей, зафиксированных формалином и заключенных в парафин. Образцы семенников обрабатывали в ксилоле и спирте для удаления парафина и проводили демаскировку антигенов при помощи нагрева на водяной бане Biosan WB-4MS в цитратном буфере с рН=6 в течение 20 мин при 97°С.
После проведения демаскировки наносили на образцы Peroxidase blocking solution (Abcam) и инкубировали 10 мин при комнатной температуре. Затем промывали образцы в PBS (фосфатно-солевом буфере) в течение 5 мин, наносили на образец Protein blocking solution (Abcam) и инкубировали 15 мин при комнатной температуре. После инкубации наносили на образцы исследуемые антитела к человеческим p53, Bcl-2, pH2AX (Ser139), PDGFRα, PCNA в разведении 1:50–1:100 и инкубировали в течение 18–20 ч при 4°С.
После инкубации образцы промывали в PBS 2 раза, наносили вторичные антитела, конъюгированные с HRP (пероксидазой хрена) и инкубировали в течение 30 мин при комнатной температуре. Далее на образцы наносили 2–3 капли раствора диаминобензидина (Abcam, ab64238) и инкубировали 5 мин при комнатной температуре. Затем срезы подкрашивали гематоксилином Майера и заключали под покровное стекло. Окрашенные клетки подсчитывали в 10 полях микроскопа при увеличении ×200 с использованием микроскопа Nikon Eclipse 80i с Ds-Fi1 (Nikon, Япония).
Статистический анализ
Данные, полученные в результате подсчета, статистически обрабатывали с помощью компьютерной программы Graph Pad Prism 8.2.1. Определяли среднее значение и стандартное отклонение. Для статистической обработки с целью оценки достоверности различий средних значений между группами использовали непараметрический U-критерий Манна–Уитни.
Результаты
Определение активности сперматозоидов
Восемьдесят самцов в возрасте 6–7 нед. были разделены на четыре группы для индукции репродуктивного старения и последующей терапии (рис. 1, раздел 2.2. «Дизайн исследования»). После завершения терапии мышей подвергали эвтаназии на 1-й, 10-й, 20-й, 30-й день и получали образцы спермы, крови и семенников.
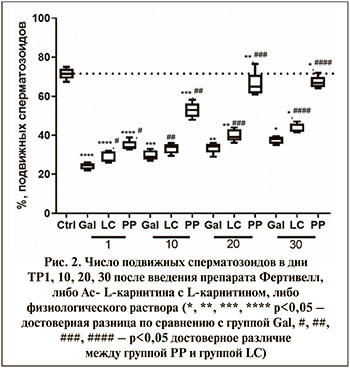
Согласно полученным результатам, у мышей интактной группы (группа Ctrl) количество подвижных сперматозоидов составляет 71,6±2,8%, что соответствует норме для мышей линии С57Вl/6J. D-галактоза вызывала снижение количества подвижных сперматозоидов до 24,2±1,6% в день ТР1, т.е. через 30 дней после окончания формирования модели старения (группа Gal). В дальнейшем происходит постепенное увеличение числа подвижных сперматозоидов, которое достигает 37,6±1,9% в день ТР30.
Фертивелл (группа PP) в дозе 1 мг/кг статистически значимо увеличивал число подвижных сперматозоидов до 34,7±2,4% по сравнению с группой LC – 28,6±2,9% (р<0,05) в день ТР1 после окончания введения. К ТР30 число подвижных сперматозоидов в группе PP увеличивается до 67,4±3,1%, что сопоставимо с уровнем интактного контроля (рис. 2).
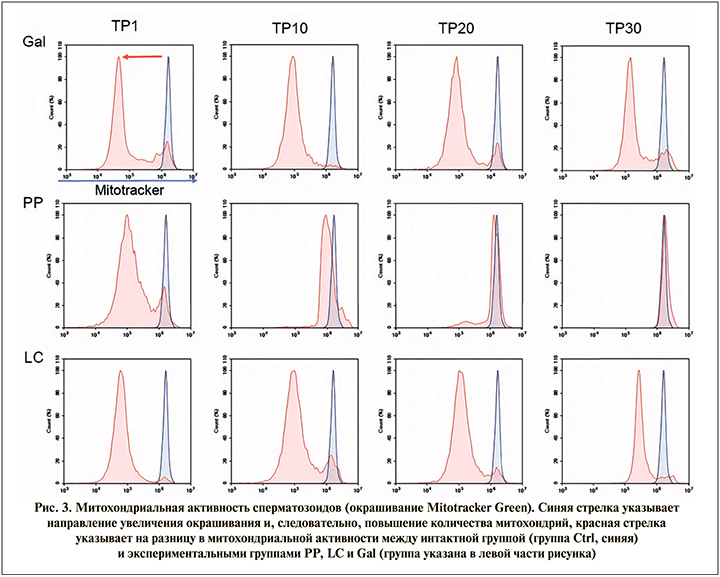
Измерение митохондриальной активности сперматозоидов с использованием окрашивания Mitotracker Green и CellRox DeepRed и анализа TUNEL
Для оценки митохондриальной активности сперматозоиды окрашивали Mitotracker Green и анализировали на проточном спектрометре. Синяя стрелка указывает направление усиления окрашивания и, следовательно, увеличение количества митохондрий. Полученные результаты показывают, что D-галактоза на модели старения значительно снижает активность митохондрий по сравнению с интактной группой (группа Ctrl), это приводило к потере подвижности сперматозоидов. Фертивелл восстанавливает активность митохондрий сперматозоидов в день ТР30 (через 30 дней после отмены терапии), это также отражается на восстановлении подвижности сперматозоидов. Применение L-карнитина лишь частично восстанавливало активность митохондрий. Использование препарата Фертивелл не только восстанавливало митохондриальную активность, но и повышало ее выше уровня контроля (рис. 3). Этот факт подтвердился при исследовании цитологических образцов сперматозоидов для оценки морфологии и расположения окрашенных органоидов сперматозоидов.
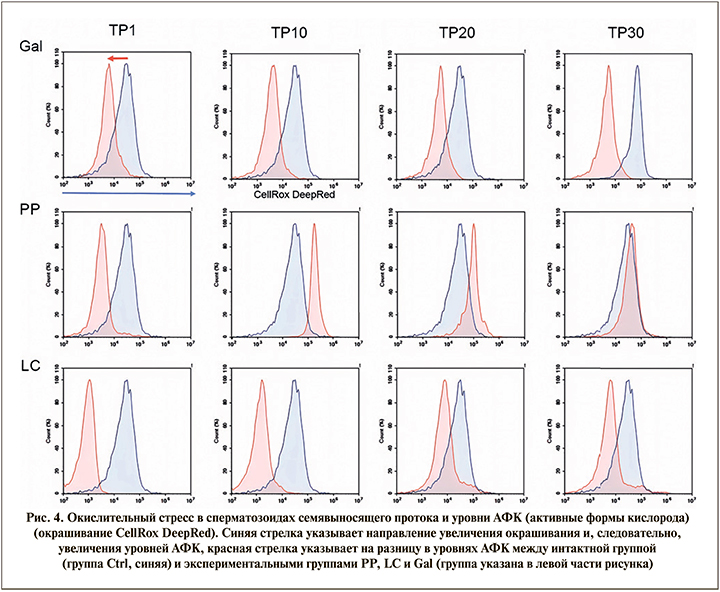
Сперматозоиды окрашивали сенсором АФК CellRox DeepRed и анализировали методом проточной цитометрии на приборе Novocyte 2000R (ACEA, США). D-галактоза значительно снижает внутриклеточный уровень АФК сперматозоидов, выделенных из семявыносящего протока, что может быть связано со снижением уровня метаболизма в сперматозоидах (в т.ч. со снижением активности митохондрий). Следует отметить, что L-карнитин вызывал более глубокое подавление образования АФК, тогда как Фертивелл восстанавливал (рис. 4) нормальный уровень АФК до значений интактной группы.
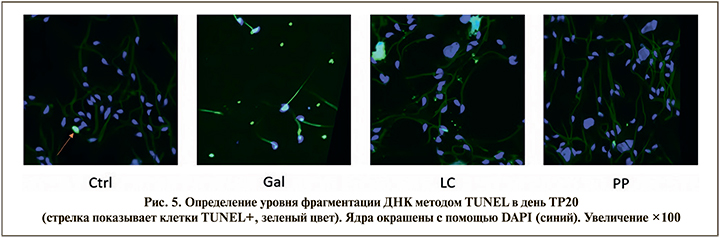
При проведении анализа TUNEL (terminal deoxynucleotidyl transferase dUTP Nick End Labeling) – оценки количества сперматозоидов с фрагментированной ДНК в день ТР20 было обнаружено (рис. 5), что D-галактоза приводит к увеличению количества TUNEL+сперматозоидов семявыносящего протока, т.е. сперматозоидов с фрагментированной ДНК до 30% по сравнению с контролем (5%). L-карнитин уменьшает количество TUNEL+клеток (15%). Фертивелл снижает количество TUNEL+клеток до уровня интактной группы (5%). Следовательно, препарат Фертивелл приводит к репарации ДНК, которая характеризуется уменьшением числа TUNEL+клеток, т.е. уровня фрагментации ДНК.
Анализ ИГХ
Белок р53 выявляется во многих трансформированных клетках, локализуется в ядре и является «аварийным тормозом пролиферации», т.е. является проапоптотическим, регулируя процессы апоптоза в сперматогониях [18]. В экспериментах на мышах при нормальном сперматогенезе р53 в сперматогониях экспрессируется слабо, появляясь более выраженно только после воздействия негативных факторов. Группа исследователей под руководством Lian показала, что повышение уровня АФК вызывает усиление экспрессии р53, что приводит к изменениям в семенных канальцах и снижению подвижности сперматозоидов [19].
Группа Gal показала увеличение экспрессии р53 (2+, >80% клеток семенных канальцев) по сравнению с группой Ctrl (1+, 30% клеток). В группах LC и PP число клеток р53+ уменьшилось (1+, <30%) ко дню ТР20 после начала терапии по сравнению с группой Gal.
Белки семейства Bcl-2 распределены в различных компартментах половых клеток и участвуют в процессах дифференцировки и созревания при сперматогенезе. Bcl-2 локализуется на внешней мембране митохондрий, где играет важную роль в метаболической защите: повышает устойчивость митохондриальных мембран, предотвращая их повреждение, повышение АФК и последующий клеточный апоптоз [20]. Также показано, что экспрессия Bсl-2 препятствует гибели сперматогоний. Экспрессию Bcl-2 обнаруживали в цитоплазме отдельных клеток Лейдига, расположенных ближе к семенным канальцам, и клетках сперматогенного эпителия, включая клетки Сертоли. Уменьшение числа клеток Bcl-2+ (1+, <30% клеток) было обнаружено в группе Gal по сравнению с группой Ctrl (2+, 60% клеток) (рис. 6). В группе LC число клеток Bcl-2+ не изменилось (1+, <30%), а в группе PP количество клеток, экспрессирующих Bcl-2, увеличилось до уровня группы Ctrl.
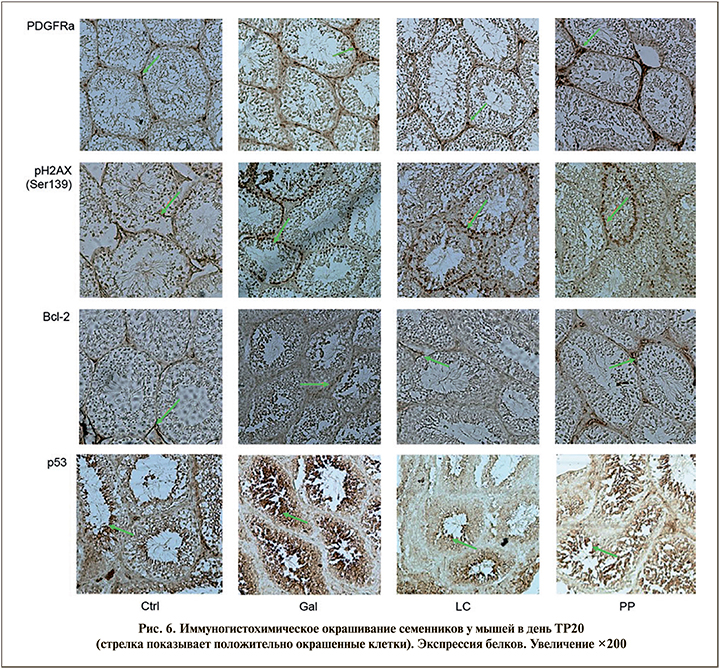
Экспрессия рецепторов к фактору роста тромбоцитов (PDGFRα) была обнаружена преимущественно в клетках Лейдига и эндотелиоцитах кровеносных сосудов в периферических семенных канальцах [21]. Фактор роста тромбоцитов (PDGF) играет важную роль в регуляции развития и функции мужских половых желез. В образцах семенников от пациентов с полной аплазией зародышевых клеток или различной степенью нарушения сперматогенеза иммуногистохимическая локализация PDGF и PDGFRα отличалась от нормальной, что подтверждает тесную связь между зародышевыми клетками и системным распределением PDGF [22].
В контрольной группе обнаружено менее <20% клеток с интенсивностью окрашивания 1+. В группе Gal было обнаружено небольшое увеличение PDGFRα+клеток (1+, <30% клеток). В группе LC клетки PDGFRα+ были сопоставимы с контролем (1+, <30% клеток). В группе PP число клеток PDGFRα+ не только восстанавливается, но и увеличивается вместе с интенсивностью окрашивания (3+, >80%) ко дню TP30 после введения препарата Фертивелл, что свидетельствует об усилении экспрессии рецепторов к фактору роста тромбоцитов и, соответственно, об активации клеток Лейдига и нормализации процесса сперматогенеза.
Двухцепочечные разрывы (повреждение) ДНК всегда сопровождаются фосфорилированием гистона γ-H2AX Ser139, компонента октамера гистонов в нуклеосомах. Увеличение числа клеток γ-H2AX Ser139 коррелирует с увеличением количества клеток с поврежденным ДНК (3+, >70% клеток). В контрольной группе обнаружены единичные клетки Сертоли, окрашенные антителом к γ-H2AX Ser139. В группах PP (1+, <40%) и LC (2+, <50% клеток) количество клеток γ-H2AX Ser139 уменьшилось в день TP30 после начала приема препаратов по сравнению с группой Gal, что также свидетельствует о снижении уровня фрагментации ДНК не только в сперматозоидах, но и в клетках Сертоли.
Анализ содержания тестостерона в образцах
Концентрация тестостерона плазмы, полученная от самцов мышей линии C57BL/6J, составила 2,42±0,75 нМ/мл (0,8±0,2 нг/мл), что соответствует уровню тестостерона, определяемому радиоиммунологическим методом у здоровых половозрелых (8–11 мес.) и стареющих (29–31 мес.) самцов мышей линии C57BL/6J. Медианный и 95-процентильный уровни тестостерона в плазме в нг/мл составляли 1,12 (0,19–12,18) у половозрелых мышей и 1,17 (0–7,31) у стареющих мышей [23]. D-галактоза приводит к статистически значимому (p<0,05) 3-кратному снижению уровня тестостерона в плазме (0,82±0,23 против 2,42±0,75 нМ/мл) в день TP1, т.е. через 30 дней после прекращения введения D-галактозы. В дальнейшем мы наблюдали тенденцию к восстановлению уровня тестостерона в плазме, достигавшего значений 1,47±0,46 нМ/мл (60,7% от контрольной группы) ко дню ТР30. Введение препарата Фертивелл (1 мг/кг) позволяет добиваться восстановления уровня тестостерона в плазме ко дню ТР20 – 1,97±0,54 (81,4% от контрольной группы) и сохранения прироста ко дню ТР30 после окончания введения препарата – 2,23±0,75 нМ/мл (92,1% от контроля).
Статистически значимая разница между достигнутыми уровнями тестостерона в плазме крови в группах LC и PP наблюдалась на 20-й день, к 30-му дню тенденция сохранялась. Статистически значимые различия установлены по отношению к группе Ctrl (р<0,05).
Экспрессия мРНК ключевых белков сперматогенеза
Гексокиназа (Hk1, EC2.7.1.1) является начальным ферментом гликолитического пути и использует АТФ для фосфорилирования глюкозы и производства глюкозо-6-фосфата (G6P), первого шага в большинстве путей метаболизма глюкозы. Hk1 локализован в основной части жгутика сперматозоидов на наружной мембране митохондрий, где были обнаружены другие специфичные для сперматозоидов гликолитические ферменты.
Было обнаружено, что введение D-галактозы значительно снижает экспрессию мРНК белка Hk1. Введение препарата Фертивелл вызывало восстановление экспрессии белка Hk1, достигая максимума в день ТР1 после окончания введения и снижаясь ко дню ТР30. L-карнитин не вызывал восстановления экспрессии генов до уровня группы Ctrl в течение всего времени.
Белок-переносчик фосфолипидов (PLTP) опосредует перенос фосфолипидов и свободного холестерина из липопротеинов, богатых триглицеридами (липопротеинов низкой плотности (ЛПНП) и липопротеинов очень низкой плотности (ЛПОНП)) в липопротеины высокой плотности (ЛПВП), а также обмен фосфолипидов между липопротеинами, богатыми триглицеридами. Дефицит PLTP ассоциирован с гипофертильностью у самцов мышей.
Введение D-галактозы незначительно снижало экспрессию мРНК Pltp по сравнению с уровнем интактного контроля (p>0,05). Введение как Фертивелла, так и препарата сравнения L-карнитина приводило к восстановлению и сопоставимому увеличению экспрессии мРНК Pltp, достигая максимума в день TP30 после окончания введения, разница между группами не была достоверной.
Пероксиредоксин-1 (Prdx1) представляет собой тиолспецифическую пероксидазу, которая катализирует восстановление перекиси водорода и органических гидроперекисей до воды и спиртов соответственно. Он играет роль в защите клеток от окислительного стресса путем детоксикации перекисей и в качестве сенсора сигнальных событий, опосредованных перекисью водорода.
Было обнаружено, что введение D-галактозы не приводило к статистически значимому изменению экспрессии Prdx1 по сравнению с уровнем контрольной группы (p>0,05). Введение препарата Фертивелл и препарата сравнения L-карнитина вызывало статистически значимое увеличение экспрессии Prdx1 (p<0,05).
Hspa5 (шаперон BiP (GRP78)) представляет собой шаперон эндоплазматического ретикулума – специальный белок, который играет ключевую роль в фолдинге (процессе «укладки» белка, приобретении им правильной конформации) и контроле качества белков в просвете эндоплазматического ретикулума. Grp78 участвует в правильном фолдинге белков и деградации неправильно свернутых белков. В сперматозоидах Grp78 обнаружен в пришеечной области.
D-галактоза статистически значимо не изменяет экспрессию мРНК Hspa5 по сравнению с уровнем группы Ctrl (p>0,05). Фертивелл и препарат сравнения L-карнитин вызывают статистически значимое увеличение экспрессии Hspa5 (p<0,05) – прирост уровня превышал значение в группе Ctrl.
Экспрессия белка виментина наблюдалась только в клетках Сертоли, что указывает на специфическое расположение филаментов в зависимости от стадии. Виментиновые филаменты клеток Сертоли играют важную роль в поддержании и регуляции этапов сперматогенеза: их повреждение связано с распадом тестикулярного эпителия, а восстановление – с восстановлением сперматогенеза после исчезновения неблагоприятных условий. D-галактоза не вызывала статистически значимого изменения экспрессии мРНК виментина по сравнению с уровнем контрольной группы (p>0,05). Фертивелл и препарат сравнения L-карнитин вызывают статистически значимое увеличение экспрессии виментина.
Обсуждение. На сегодняшний день выбор доклинических моделей для воспроизведения естественного репродуктивного старения достаточно ограничен: введение бусульфана или производных нитрозомочевины, применение гаммаизлучения, при этом каждая из вышеперечисленных более отдалена от естественного старения, чем модель на основе длительного введения D-галактозы – она также не полностью отражает развитие естественного репродуктивного старения и учитывает лишь некоторые его механизмы, но в то же время совокупность повреждающих факторов приводит к схожим изменениям.
Окислительный стресс играет ключевую роль в формировании возрастных изменений и развитии бесплодия. В норме система антиоксидантных ферментов (одним из них является пероксиредоксин-1, рис. 7) поддерживает оптимальную концентрацию свободных радикалов в организме, но высокие уровни АФК нарушают баланс и приводят к окислительному стрессу в репродуктивных тканях. При окислительном стрессе липиды в клетках Лейдига или Сертоли подвергаются перекисному окислению, что в итоге приводит к повреждению клеточной ДНК и постепенной утрате функции клеток и, соответственно, органов. В результате окислительный каскад приводит к снижению продукции тестостерона и нарушениям процесса сперматогенеза, что является одним из основных механизмов развития мужского бесплодия. Интересно отметить, что уменьшение числа двухцепочечных разрывов ДНК под влиянием препарата Фертивелл происходит не только в сперматозоидах семявыносящего протока, но и в клетках Сертоли в яичках, что имеет большое значение для нормализации локальной регуляции процессов сперматогенеза, осуществляемой клетками Сертоли.
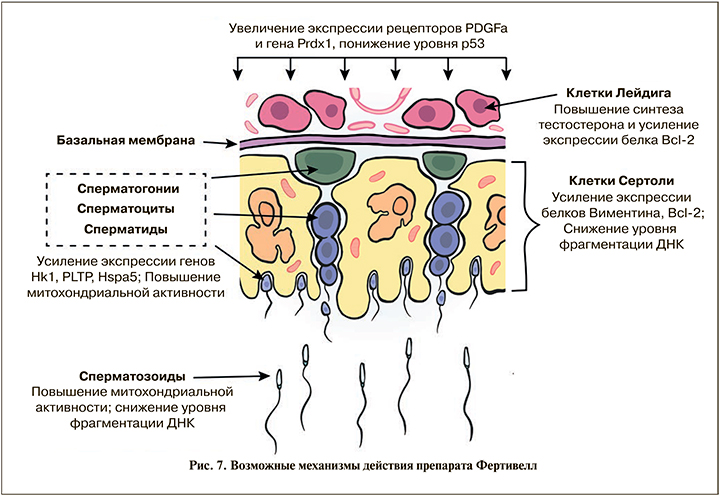
Тестостерон глубоко вовлечен в каждый этап физиологических процессов в мужской репродуктивной системе.
Помимо центральной регуляции продукции тестостерона и интенсивности сперматогенеза в семенниках лютеинизирующим и фолликулостимулирующим гормонами, существует локальная, внутритестикулярная система регуляции, которая осуществляется за счет факторов, синтезируемых клетками Сертоли. Таким образом, клетки Сертоли, взаимодействуя с клетками Лейдига, регулируют функциональную активность семенников, в том числе обеспечивая критические факторы, необходимые для успешного развития половых клеток в сперматозоиды. Важно отметить, что клетки Лейдига, основной источник тестостерона в организме, также подвержены окислительному стрессу, и уровень их окисления можно измерить по скорости экспрессии различных факторов, таких как Bcl-2.
Чтобы быть функциональными, сперматозоиды должны пройти созревание в придатке семенников, капацитацию и акросомную реакцию. Эти события созависимы, так как акросомная реакция не возникает, если капацитация нарушена, а капацитация происходит только при наличии подвижных зрелых сперматозоидов. Для обеспечения подвижности сперматозоидов необходимо поддержание энергетических каскадов, включающих запуск процесса гликолиза и перенос молекул АТФ из митохондрий в жгутик. Для оценки способности сперматозоидов к движению существуют лабораторные методы оценки митохондриальной активности или уровня экспрессии определенных генов, таких как Hk1 или Hspa5 (GRP78).
Выводы. Препарат на основе полипептидов семенников Фертивелл оказывает комплексное действие на репродуктивную функцию, приводя к изменению экспрессии генов, повышению синтеза белков, предотвращению повреждений ДНК в тканях семенников, повышению митохондриальной активности в тканях семенников и сперматозоидах семявыносящего канальца, что приводит к последующему восстановлению функциональной активности семенников.
Наблюдается влияние препарата в отношении как экзокринной функции семенников или сперматогенеза – повышение количества живых сперматозоидов, повышение количества активно-подвижных форм, так и эндокринной функции, что проявляется в повышении синтеза тестостерона, повышении экспрессии белков клетками Лейдига.
Примечательно, что препарат на основе полипептидов Фертивелл оказывает выраженное действие на клетки семенников и сперматозоиды в экспериментальной модели in vivo и показал себя более эффективным протектором от окислительного стресса по сравнению с L-карнитином, широко используемым в качестве метаболического агента для лечения мужского бесплодия. Однако в отличие от терапии L-карнитином под влиянием препарата Фертивелл происходит восстановление уровня АФК до нормальных значений, не приводя к его избыточному подавлению, что важно для обеспечения энергетических процессов, процесса сперматогенеза и нормальной подвижности сперматозоидов.



