Введение. Биологическая состоятельность клетки человека зависит от условий непосредственного окружения и непрерывности снабжения питательными веществами, прежде всего глюкозой и липидами, в условиях эффективного удаление продуктов их метаболизма [1, 2]. При этом целостность клеточной мембраны поддерживается постоянным обновлением липидного бислоя, в котором преобладают фракции фосфолипидов. Энергозависимые белковые «насосы», встроенные в плазматическую мембрану, насыщают клетку энергоемкими молекулами глюкозы, формирующими «потенциал действия» для выполнения специфических функций конкретной генетически детерминированной ткани [3, 4]. Структурно состоятельная клетка, отключенная от энергообеспечения, теряет важнейшую функциональную потенцию к обновлению липидного бислоя мембран, что приводит к снижению ее коммуникационной способности [5].
Известно, что ведущими морфологическими признаками первичного хронического пиелонефрита на ранних стадиях формирования является появление диффузной лимфогистиоцитарной инфильтрации с разрастанием в интерстициальной зоне соединительной ткани, сужением просвета канальцев, утолщением стенок артериол с уменьшением размеров клубочков, что связано со структурно-функциональной несостоятельностью цитомембран в условиях нарушения липидного и энергетического метаболизма клеток [6, 7].
Ведущим фактором формирования хронического бактериального цистита является микробный патоген. Он повреждает уротелий и провоцирует его метаплазию. Все дальнейшие изменения в стенке мочевого пузыря происходят вне зависимости от бактериального начала. Они характеризуются сочетанием деструктивных и компенсаторно-приспособительных реакций, чередованием дистрофических изменений и метаплазии с увеличением пролиферативной активности и фокальной альтерации [8, 9]. Основу позднего постлучевого воспаления в строме мочевого пузыря составляет нарушение тканевого гомеостаза, не позволяющего завершить хроническое воспаления регенерацией. При этом периоды затихания патологического процесса прерываются обострением, что фиксируется в процессе морфологического анализа и объясняется проявлением вторичной пероксидацией мембранных липидов [10, 11].
Стандартная совмещенная позитронно-эмиссионная и компьютерная томография (ПЭТ/КТ) всего тела человека независимо от показаний к его проведению позволяет визуализировать и количественно рассчитывать в процессе одного исследования физиологическую, функциональную и, возможно, патологическую составляющие анатомо-метаболического состояния органов конкретного человека [12, 13], При этом тропность тканей к биомолекулам 18F-ФДГ глюкозы отражает состоятельность энергетической потенциала органов всего тела обследуемого человека и локального в регионе интересов лечащего врача [14–18]. Сканирование с предшественником мембранных фосфолипидов 11С-холином регистрирует активность липидного метаболизма как всего тела, так и локального в области выявленных изменений [19–25]. Нарушения тканевого обмена в процессе реализации хронического воспаления проявляются либо функциональным метаболическим «оглушением», либо гипер-, либо гипометаболизмом тканей, что зависит от активности и локализации процесса.
И это можно не только увидеть в режиме реального времени, но и рассчитать количественно, что не доступно другим существующим методам диагностики [26, 27]. Вместе с тем в научной литературе крайне мало работ, посвященных применению ПЭТ/КТ-сканирования в диагностике неонкологических воспалительных заболеваний органов мочевой системы, что стало предметом специального изучения.
Цель исследования: определение клинической значимости ПЭТ/КТ-сканирования органов мочевой системы в молекулярно-клеточной диагностике воспалительных заболеваний органов мочевой системы.
Материалы и методы. Для достижения поставленной цели с 2018 по 2022 г. проведено сравнительное изучение результатов ПЭТ/КТ всего тела человека, а также морфобиопсии почек и мочевого пузыря у 96 пациентов радиологического центра и отделений урологии Тюмени, среди которых было 56 женщин и 40 мужчин с медианой возраста 51,5 (37;61). В процессе проведения исследования 48 пациентов прошли сканирование с предшественником фосфолипидов 11С-холином и 48 с энергоемкими молекулами 18F-ФДГ глюкозы. Больные были информированы об участии в клиническом исследовании по поиску оптимального режима проведения ПЭТ/КТ, позволяющего более точно оценивать анатомо-метаболическое состояние их органов, на что были получены письменные согласия.
Оценку состояния энергетического метаболизма всего тела пациентов выполняли методом ПЭТ/КТ на аппарате Biograph («Siemens»). Анализ данных проводили визуальным методом с 3D-реконструкцией изображения. Изучали активность метаболизма 11С-холина или 18F-ФДГ глюкозы в зонах интереса, выделенных штрих-линией по уровню захвата изотопа (SUVmax), в рамках физиологических отклонений показателей от 3,0 до 10,5 гр/л. Средняя доза однократно вводимого внутривенно 5 мл 11С-холина составила 600 МБк. Исследования проводили в течение 20 мин через 5 мин после введения препарата. ПЭТ/КТ с 18F-ФДГ проводили в течение 30 мин через 20 мин после внутривенного ведения 5 мл (200 МБк) препарата. Препараты готовились в Тюменском радиологическом центре на компактном циклотроне («Scanditronix»). Анатомо-морфологические исследования состояния паренхимы почек и слизистой мочевого пузыря выполнены в патологоанатомическом бюро ГАУЗ ТО МК МЦ «Медицинский город» Тюмени по стандартной методике. Морфологический материал, полученный в процессе пункционной биопсии, проведенной под ультразвуковой и ПЭТ/КТ-навигацией в стационарных условиях, фиксировали в нейтральном формалине, обезвоживали и заливали в парафин. Срезы толщиной 3–5 мкм окрашивали гематоксилин-эозином. Обзорное гистологические исследование проводили при увеличении х40. Статистический анализ данных проводили согласно международным требованиям, предъявляемым к обработке данных научных исследований, при помощи программы для персональных компьютеров Statistica for Windows (версия 11.5). Непрерывные переменные представлены в виде M±m (среднее±стандартная ошибка среднего). Статистическую значимость различий оценивали по t-критерию Стьюдента.
Результаты. На первом этапе совместно с врачом-радиологом отбирались томограммы пациентов без анамнестических и лабораторно-инструментальных проявлений урологических заболеваний с наиболее высокими визуальными и количественными показателями липидного или энергетического метаболизма, которые были приняты за условную норму. Полученные результаты сопоставляли с морфологического картиной биоптатов паренхимы почек и стенки мочевого пузыря с минимальными ишемическими изменениями, забранных в процессе хирургического лечения травматических повреждений в быту у лиц без отягощенного нефроурологического анамнеза (рис. 1).
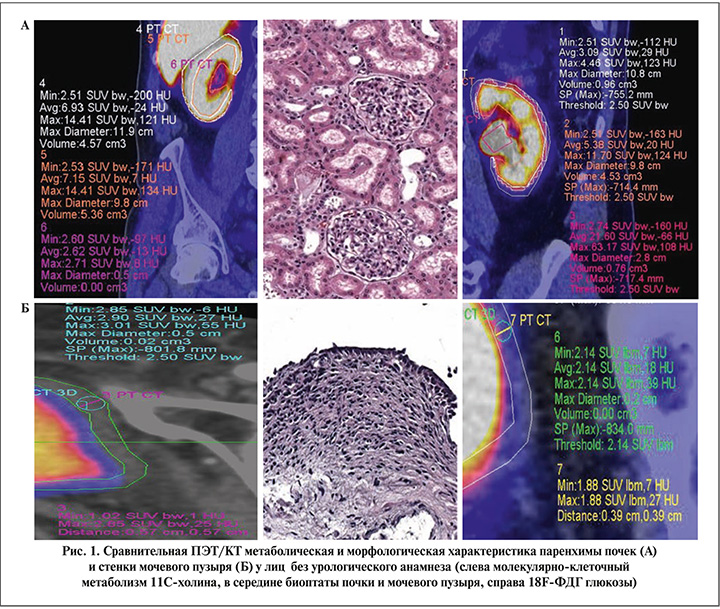
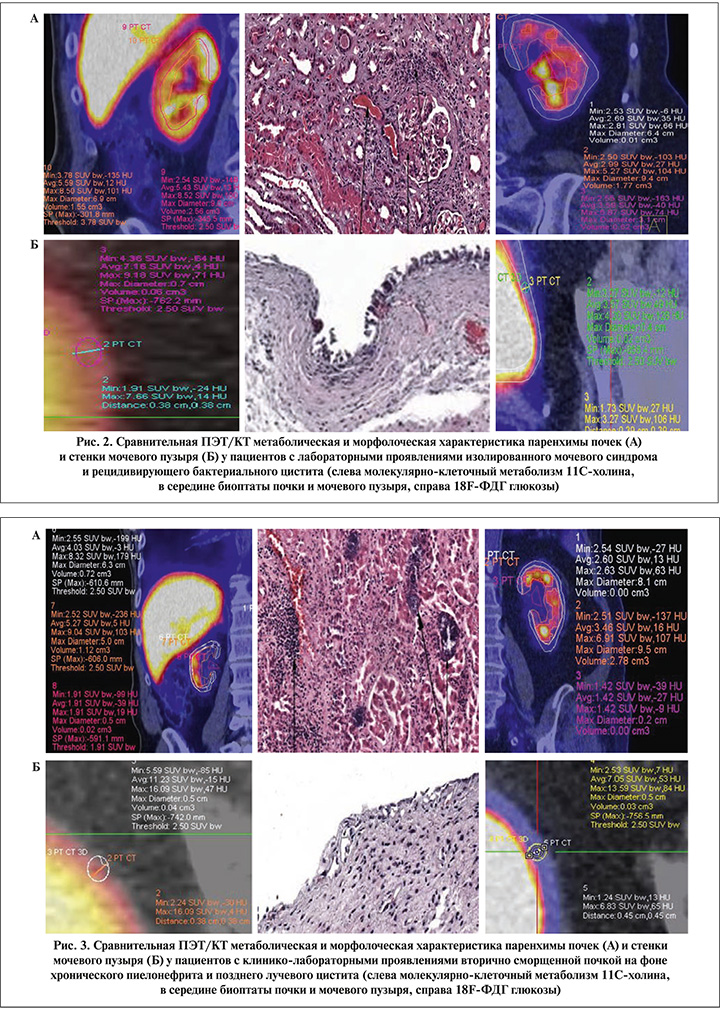
Следующую группу составили пациенты с впервые выявленным изолированным мочевым синдромом без четких клинических проявлений нефроурологических заболевани, которым перед биопсией для исключения возможных онкологических причин возникновения мочевого синдрома инструментальные методы исследования дополнялись ПЭТ/КТ-сканированием всего тела (рис. 2).
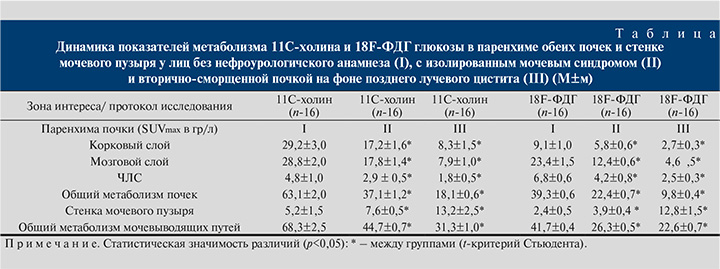
На следующем этапе пациентам с вторично сморщенными почками и значительным снижением тропности паренхимы к 11С-холину или 18F-ФДГ глюкозе проводились сравнительные исследования в сопоставлении с результатами нефробиопсии, а среди пациентов с манифестацией позднего лучевого цистита с проявлениями гиперметаболизма этих биомолекул с результатами прицельной биопсии слизистой мочевого пузыря (рис. 3).
Результаты количественного цифрового анализа тропности тканей паренхимы почек и стенки мочевого пузыря в соизмеримых объемах регионов интереса позволили установить наличие синхронного снижения метаболизма 11С-холина и 18F-ФДГ глюкозы в паренхиме почек и достоверный рост содержания меченых биомолекул в стенке мочевого пузыря, что коррелировало с выраженностью морфологических проявлений патологических изменений (см. таблицу).
Обсуждение. На современном этапе инструментальных исследований только высокотехнологичная наукоемкая процедура ПЭТ/КТ молекулярно-клеточного сканирования позволяет визуально и количественно рассчитывать метаболическую жизнеспособность тканей как всего тела, так и региона интереса лечащего врача. В результате настоящего исследования выявлено синхронное снижение метаболизма 11С-холина и 18F-ФДГ глюкозы в паренхиме почек и достоверный рост липидного и углеводного обменов в стенке мочевого пузыря, которые коррелируют с выраженностью морфологических проявлений патологических изменений и указывают на наличие особенностей молекулярно-клеточного метаболизма органов верхних и нижних мочевыводящих путей в норме и в процесс реализации хронического воспаления тканей, что нуждается в дальнейшем специальном изучении и анализе.
Заключение. Представленные в настоящей работе результаты поисковых исследований отражают актуальность анализируемой проблемы для клинической урологии. Они раскрывают недооцененные возможности уже широко внедренной, но ограниченной только онкоурологией уникальной ядерной технологией. Результаты настоящего исследования позволяют предложить клиницистам интегративный подход к возможностям этого вида высокотехнологичного исследования с получением результатов в режиме реального времени.



