Введение. Симптомы нижних мочевыводящих путей (СНМП) у мужчин чаще всего вызваны механической обструкцией. Крайнее проявление СНМП – острая задержка мочи, риск развития которой увеличивается с возрастом [1]. Так, после 40 лет доброкачественная гиперплазия предстательной железы (ДГПЖ) как наиболее частая причина инфравезикальной обструкции встречается у 25% мужчин, после 50 лет – у 32%, после 60 лет аденомой страдают уже 84% мужчин [2]. К основным вариантам оперативного лечения доброкачественной гиперплазии предстательной железы (ДГПЖ) в настоящее время относятся открытая аденомэктомия и трансуретральная резекция (ТУР) предстательной железы [3], однако зачастую эти методы противопоказаны больным сердечно-сосудистой патологией, сахарным диабетом и другими сопутствующими заболеваниями. Один из новых методов лечения заболевания – эндоваскулярная эмболизация артерий простаты (ЭАП), результаты использования которого были опубликованы в 2010 г. J. M. Pisco et al. [4]. Метод оказался технически успешным для 14 (93,3%) из 15 пациентов.
У 30 катетерзависимых пациентов с большими размерами предстательной железы и серьезной сопутствующей патологией, прооперированных по данной методике, отмечено уменьшение размеров предстательной железы и улучшение качества жизни по шкале IPSS–QoL [5]. В России в 2010 г. также были представлены данные об ЭАП у 40 пациентов с высоким риском стандартного оперативного вмешательства. Эмболизация артерий предстательной железы помимо снижения выраженности клинических проявлений позволила уменьшить объем предстательной железы на 50% [6], отдельные авторы свидетельствуют также об улучшении показателей уродинамики [7]. В настоящее время ЭАП не является методом выбора в лечении пациентов с ДГПЖ, в связи с чем актуальны исследования возможностей его применения, в том числе по сравнению с чреспузырной аденомэктомией и трансуретральной резекцией.
Цель исследования: оценить возможности применения ЭАП в лечении пациентов с ДГПЖ в различных возрастных группах в зависимости от степени выраженности симптомов нарушенного мочеиспускания, данных УЗИ простаты, степени операционного риска по шкале ASA.
Материалы и методы. В 2016 г. в условиях хирургического отделения клиники проведено обследование и лечение 39 пациентов с ДГПЖ 2–3-й ст. Протокол клинического исследования был утвержден ученым советом и одобрен локальным этическим комитетом НИИКЭЛ – филиал ИЦиГ СО РАН (протокол № 155 от 24.12.2015). С целью персонификации лечения проведена оценка операционных рисков по шкале ASA.
До операции, через 3 и 6 мес. после нее проведена оценка выраженности симптомов нижних мочевыводящих путей по шкале IPSS, а также трансректальное УЗИ (ТРУЗИ) простаты с оценкой объема остаточной мочи.
На этапе скрининга всем пациентам с уровнем простатспецифического антигена (ПСА) более 4 нг/мл проведена биопсия предстательной железы. В исследование включили только пациентов с ДГПЖ.
Пациенты были разделены на 3 группы: пациентам первой группы (n=12) была выполнена классическая чреспузырная аденомэктомия «вслепую» (ЧПА); второй (n=19) – ТУР предстательной железы биполярной энергией на оборудовании фирмы «Karl Storz». Операции выполнены под спинальной анестезией со стандартной профилактикой тромбоэмболических осложнений. Пациентов активизировали на следующие сутки после хирургического вмешательства.
Пациентам третьей группы (n=8) была выполнена ЭАП. После обработки операционного поля под местной анестезией осуществлен доступ в правую общую бедренную артерию по Сельдингеру с установкой интродьюсера 5 Fr. Далее проведена катетеризация правой внутренней подвздошной артерии с помощью гидрофильного проводника и катетера Cobra. С помощью микрокатетера Progreat 2,9–2,4 Fr, гидрофильного проводника 0,014 и 0,018 выполнена катетеризация правой нижней пузырной артерии и ее простатической ветви, а затем эмболизация введением Embosphere 300–500 мкм. Затем по аналогии проведена катетеризация левой нижней пузырной артерии и ее простатической ветви с эмболизацией последней. После эмболизации осуществлен контроль периферического кровотока в предстательной железе, отсутствие которого свидетельствовало об успешности манипуляции. Гемостаз в области установки интродьюсера семи пациентам осуществлен с помощью сшивающей системы Exoseal, одному пациенту – путем наложения давящей повязки на область пункции бедренной артерии.
Распределение пациентов в одну из трех групп (т.е. планирование одной из трех операций) происходило с учетом объема предстательной железы и наличия сопутствующих хронических заболеваний. В группу ЧПА вошли пациенты с анестезиологическим риском I–II по ASA, в группу ТУР – с риском II по ASA, в группу ЭАП – с риском III.
Пациентам группы ЧПА мочевой катетер удален на 5–7-е сутки, группы ТУР – на 4–5-е, группы ЭАП – вечером в день операции пациентов активизировали.
Количественные данные, полученные в работе, были обработаны с помощью общепринятых методов системного анализа с использованием электронных таблиц Excel и пакета прикладных программ Statistica 10 Eneterpise, версия 10.0.228.8. Применены методы непараметрической статистики: корреляционный анализ Спирмена, тест Манна–Уитни при сравнении групп и тест Вилкоксона для сравнения показателей в динамике внутри каждой группы. При проверке статистических гипотез критический уровень значимости принят равным 0,05. Данные представлены как медиана (25-й перцентиль; 75-й перцентиль).
Результаты. Пациенты трех групп исходно статистически значимо не различались по возрасту и уровню ПСА. Наименьшая продолжительность операции отмечена в группе ТУР, в группах ЧПА и ЭПА данный показатель достоверно не различался. Отмечается положительная корреляция объема предстательной железы и объема остаточной мочи. Наибольший объем предстательной железы отмечен в группе ЧПА. При этом по шкале IPSS между группами не отмечено достоверных различий, что свидетельствует о том, что выраженность симптомов нижних мочевыводящих путей не зависит от объема предстательной железы. Наибольший койко-день отмечен в группе ЧПА, что связано с объемом операции и необходимостью более длительной катетеризации мочевого пузыря в послеоперационном периоде. Наименьший срок госпитализации был в группе ЭПА, так как операция выполняется под местной анестезией и катетеризации мочевого пузыря в послеоперационном периоде не требуется.
Характеристика пациентов, включенных в разные группы исследования, представлена в таблице.
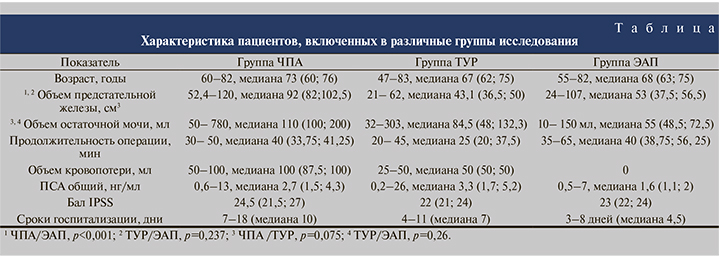
В группе ЧПА через 3 мес. после операции, по данным УЗИ, на месте удаленной аденомы определился сформированный «предпузырь», через 6 мес. определилась сформированная часть простатического отдела уретры. Через 3 мес. после операции отмечено достоверное снижение индекса IPSS с 24,5 (21,5; 27) до 13 (13,8; 16,3) баллов (p=0,002), через 6 мес. – до 9 (8,8; 10) баллов (p=0,002). Объем остаточной мочи через 3 мес. составил 2,5 (0; 10) мл (p=0,003), через 6 мес. – 0 (0; 0) мл (p=0,003).
В группе ТУР через 3 мес. после операции, по данным УЗИ, определилась сформированная простатическая часть уретры, через 6 мес. данные УЗИ не различались. По прошествии 3 и 6 мес. после операции констатировали снижение балла IPSS с 22 (21; 24) до 11 (10; 12) и до 8 (7; 9) баллов соответственно (p=0,0001 в обоих случаях). Также зафиксирована положительная динамика объема остаточной мочи, который спустя 3 мес. составил 10 (6,5; 15) мл, через 6 мес. – 0 (0; 5) мл (p=0,0001 в обоих случаях).
В группе ЭАП через 3 мес. после операции, по данным УЗИ, объем простаты снизился в среднем на 10–15%, через 6 мес. – на 15–20%, варьируясь в пределах от 35 до 92 см3. Отмечено достоверное снижение балла IPSS с 23 (22; 24) до 18 (17; 19) и 12 (11; 13) по прошествии 3 и 6 мес. соответственно (p=0,01 для обоих сроков). Объем остаточной мочи к концу срока наблюдения составил 25 (18; 30,5) мл, показав достоверное снижение (p=0,01).
При сравнении результатов лечения в трех группах выявлено достоверное отличие индекса IPSS, полученного через 3 и 6 мес. (p<0,0001 при сравнении групп ЧПА и ТУР и p=0,0003 при сравнении групп ЭАП и ЧПА для обоих сроков наблюдения; рис. 1).
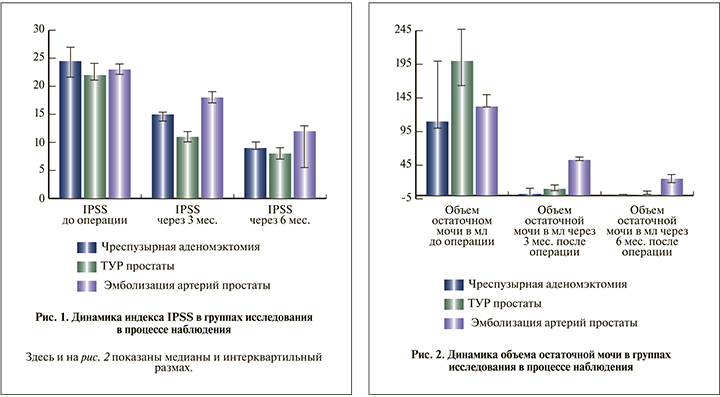
Объем остаточной мочи, измеренный через 3 и 6 мес. после операции в группах ЧПА и ТУР, достоверно не различался, в группе ЭАП через 6 мес. был выше, чем в 2 других группах (p=0,004; рис. 2).
Исходный объем железы коррелировал с исходным объемом остаточной мочи (r=0,36, p=0,03). Исходный балл IPSS достоверно коррелировал с исходным объемом остаточной мочи (r=0,4, p=0,014). Кроме того, балл IPSS, полученный через 3 мес. после операции, прямо коррелировал с исходным объемом железы (r=0,5, p=0,001) и обратно – с исходным уровнем ПСА (r=-0,39, p=0,03). Значения по шкале IPSS, полученные до операции, через 3 и 6 мес. после нее, высоко коррелировали между собой (p<0,0001).
Не обнаружено корреляции возраста пациентов с какими-либо из указанных выше параметров.
Наиболее быстрое снижение индекса IPSS происходило в группе ТУР. Это связано с малоинвазивностью вмешательства (проводится удаление, как правило, средней доли аденомы, что способствует раннему восстановлению мочеиспускания) и более ранним удалением катетера Фоли. Наименьшие показатели индекса наблюдались через 6 мес. после операции, что соответствует срокам эпителизации раневой поверхности после ТУР. В группе ЧПА снижение индекса IPSS происходило медленнее, что связано с исходно большими размерами железы пациентов этой группы, травматичностью операции, более поздним удалением катетера Фоли. Однако через 6 мес. после операции различия в количественных показателях индекса IPSS в группах ЧПА и ТУР были статистически не значимы. В группе ЭАП снижение индекса IPSS происходило наиболее медленно, что объясняется постепенным развитием асептического некроза, в дальнейшем – склероза простаты. Однако по прошествии 6 мес. после ЭАП индекс IPSS в группе уменьшился вдвое, хотя и оказался достоверно более высоким, чем в остальных группах.
Различия в динамике снижения объема остаточной мочи в трех группах также объясняются техническими особенностями вмешательств. Через 6 мес. после операции в группе ЧПА количество остаточной мочи колебалось в диапазоне от 0 до 10 мл, что связано с радикальностью операции – полным удалением аденомы. В группе ТУР объем остаточной мочи тоже резко снизился по сравнению с исходным и составил от 0 до 20 мл. В группе ЭАП снижение объема остаточной мочи было наименьшим по сравнению двумя другими группами. Такая медленная динамика служит следствием постепенно прогрессирующего уменьшения размеров простаты за счет ее некроза и атрофии.
Обсуждение. В клинических рекомендациях Российского общества урологов упоминание о эмболизации простаты как о методе выбора хирургического лечения ДГПЖ отсутствует [8]. Однако о селективной эмболизации артерий простаты как об экспериментальном методе имеются многочисленные публикации как в отечественной, так и зарубежной литературе. Так, в работе К. П. Мельника и соавт. приведены предварительные итоги лечения ДГПЖ методом суперселективной эмболизации артерий простаты микросферами 100–300 мкм у двух пациентов с ДГПЖ больших размеров [9]. В описанных наблюдениях отмечено уменьшение размера предстательной железы уже в первый месяц после выполнения операции и улучшение качества жизни в плане мочеиспускания.
По данным МРТ таза, определены зоны-полости асептического некроза во всем объеме паренхимы органа, благодаря чему впоследствии развился склероз простаты, приведший к ее уменьшению и увеличению просвета мочеиспускательного канала [9]. В большинстве работ ЭАП предлагается как альтернатива традиционным методам хирургического лечения ДГПЖ при наличии тяжелой сопутствующей сердечно-сосудистой или легочной патологии [10–12]. Так, в процессе 4,5-месячного наблюдения у 3 пациентов с тяжелой соматической патологией после ЭАП констатировали положительную динамику: уменьшилась частота мочеиспусканий, увеличился напор струи мочи, мочеиспускание стало более свободным, более редко отмечалось ощущение неполного опорожнения мочевого пузыря, снизилась частота ночных мочеиспусканий. По результатам обследования определено уменьшение объема простаты, снижение уровня ПСА [13]. Степень регресса клинических проявлений ДГПЖ, объема простаты и отсутствие серьезных осложнений предполагают уникальную роль ЭАП в лечении больных высокого хирургического риска. Отсутствие клинического эффекта большинства унилатеральных эмболизаций, связанных со сложной анатомией ветвей внутренних подвздошных артерий, указывает на необходимость всестороннего предоперационного анализа дополнительных методов диагностики и корректного отбора пациентов для данного вида вмешательства [14].
Несмотря на то что Международным обществом интервенционной радиологии ЭАП рассматривается как новая перспективная эффективная методика лечения больных ДГПЖ, характеризующаяся высоким профилем безопасности, для рекомендации ее как рутинного метода лечения требуется дальнейшее скрупулезное изучение непосредственных и отдаленных результатов лечения [15].
Заключение. Эмболизация артерий простаты может выполняться пациентам любого возраста с любым объемом предстательной железы, однако она сопряжена с достоверно более медленным снижением балльной оценки по шкале IPSS и объема остаточной мочи, а также достоверно более высокими значениями этих показателей через 6 мес. после операции. Данный малоинвазивный метод лечения в перспективе может стать альтернативой ТУР и одномоментной ЧПА для пациентов с верифицированной ДГПЖ и анестезиологическим риском ASA-III. Для рекомендации данной технологии как метода выбора требуется проведение многоцентровых рандомизированных исследований на большой выборке пациентов.



