Введение. Внутреннее дренирование верхних мочевыводящих путей – широко распространенная урологическая манипуляция. Частота использования мочеточниковых стентов в современной практике возрастает вследствие применения эндоурологических, лапароскопических и робот-ассистированных высокотехнологичных вмешательств [1–4]. Наличие у пациента мочеточникового стента часто приводит к возникновению так называемых стент-ассоциированных симптомов и служит причиной повторных обращений к врачу и снижению качества жизни (КЖ) [5, 6].
На современном этапе развития медицины показатели КЖ признаны ключевым критерием, отражающим состояние здоровья и общего благополучия человека. Оценки, данные пациентами, используются как дополнительный критерий эффективности лечения [7, 8].
Инструментами оценки КЖ являются опросники, составленные согласно строгой научной методологии и прошедшие процедуру валидации. Наиболее распространены общие опросники (SF-36, EQ-5D), однако в последнее время показано, что при их использовании не учитывается специфика конкретного заболевания. Более полную информацию о пациенте помогают получить специальные опросники [8–10].
В 2003 г. Н Joshi et al. создали специальный опросник для объективной оценки КЖ и симптомов, связанных с мочеточниковым стентом, – Ureteric Stent Symptoms Questionnaire (USSQ) [11]. В течение последнего десятилетия USSQ получил международное признание, доказаны надежность, валидность и чувствительность 9 языковых версий данного опросника: англоязычной, итальянской, французской, корейской, испанской, арабской, немецкой, турецкой, индийской [11–19]. Его версии на русском языке до настоящего времени не разработано. Исходя из этого, оценка психометрических свойств русскоязычной версии USSQ представляется актуальной.
Цель исследования: валидация русскоязычной версии специфического опросника для оценки КЖ и симптомов, связанных с мочеточниковым стентом, – USSQ.
Материалы и методы
Структура опросника. USSQ (Приложение 1) содержит 40 вопросов и 2 визуально-аналоговые шкалы (ВАШ), позволяющих оценить выраженность симптомов, обусловленных стентом, и влияние их на КЖ пациента. Вопросы сгруппированы в 6 доменов:
- симптомы, связанные с мочеиспусканием (11 вопросов);
- боль (7 вопросов и 2 ВАШ);
- общее здоровье (6 вопросов);
- трудоспособность (7 вопросов);
- половая жизнь (4 вопроса);
- дополнительные проблемы (5 вопросов).
Отдельно для каждого домена рассчитывается индекс, представляющий собой сумму баллов, полученных при ответе на все вопросы домена. Показатели шкал колеблются от 1 до 10, более высокие значения соответствуют более выраженным симптомам и более низкому качеству жизни. Разработаны 2 версии опросника для тестирования пациентов со стентом и после его удаления.
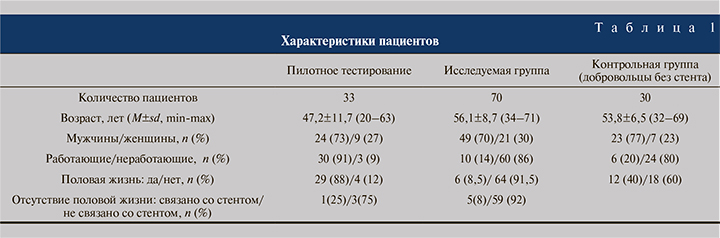
Пациенты. Исследование проведено с участием 103 пациентов, находившихся в урологическом отделении МОНИКИ с августа 2017 по декабрь 2018 г. Из них 33 пациента были включены в пилотное тестирование, 70 – составили основную группу. Контрольная группа была представлена 30 здоровыми добровольцами без мочеточникового стента. Характеристики пациентов представлены в табл. 1.
Критерии включения:
- согласие больного на участие в исследовании;
- возраст старше 18 лет;
- наличие мочеточникового катетера-стента, адекватно дренирующего верхние мочевыводящие пути, установленного по показаниям;
- отсутствие заболеваний нижних мочевыводящих путей любого генеза;
- отсутствие сопутствующих заболеваний, симптоматика которых доминирует над симптомами, обусловленными наличием стента, и требует активного лечения;
Критерии исключения:
- неспособность заполнять опросники;
- наличие осложнений оперативного лечения, требующих дополнительных вмешательств;
- билатеральное стентирование мочеточников;
- обструкция мочеточника, обусловленная злокачественным заболеванием;
- наличие мочевых дренажей другой локализации.
Пациенты самостоятельно заполняли опросник USSQ и валидные опросники EQ-5D (EUROQOL – 5D), IPSS (International Prostate Symptom Score) со шкалой QoL (Quality of Life), ВАШ боли дважды после установки мочеточникового стента с интервалом в 7 дней и через 4 нед.после удаления стента, а также отвечали на вопросы и высказывали пожелания, касавшиеся опросника, в ходе устного интервью. В первые 2 суток после установки мочеточникового стента всем пациентам выполнялись обзорная урография и УЗИ мочевыделительной системы для оценки состояния верхних мочевыводящих путей и адекватности положения стента.
Валидация опросника. Процедура валидации проводится при разработке новых опросников, а также при адаптации уже валидированных опросников к языковым и культурным особенностям той или иной страны. В последнем случае валидация состоит из следующих этапов: перевод, пилотное тестирование, оценка надежности, оценка валидности [7]. В нашем исследовании процесс валидации согласован с авторами опросника, получено их официальное разрешение на разработку и внедрение русскоязычной версии.
Культурная и языковая адаптация. На первом этапе проводился перевод опросника с английского на русский язык двумя независимыми переводчиками. После обсуждения полученных переводов и устранения различий была создана первая промежуточная версия опросника. На следующем этапе эта версия была переведена на английский язык также двумя независимыми переводчиками – 2-я промежуточная версия опросника. Окончательная корректировка проводилась при сравнении оригинальной версии со 2-й промежуточной. Выявленные неточности были устранены. В результате создана тест-версия опросника, при помощи которой проводилось предварительное (пилотное) тестирование. Его целью было провести интервьюирование небольшого количества пациентов и выяснить, насколько понятны задаваемые вопросы.
В пилотном тестировании приняли участие 33 пациента, которые охарактеризовали вопросы анкеты как понятные и четко сформулированные. Сложностей с заполнением не было ни у одного пациента. Таким образом была разработана окончательная версия опросника USSQ на русском языке.
Оценка психометрических свойств русскоязычной версии опросника USSQ проводилась с участием основной (n=70) и контрольной (n=30) групп. Определяли валидность, надежность и чувствительность русскоязычной версии опросника USSQ. Содержательная валидность определена ранее разработчиками оригинального опросника по результатам изучения данных литературы и проведения экспертной оценки в несколько этапов. В нашем исследовании содержательная валидность подтверждена группой экспертов-урологов и в процессе интервьюирования пациентов. Критериальную валидность оценивали методом «внешних критериев»: определяли корреляционные связи между суммой баллов отдельных доменов USSQ с соответствующими шкалами валидных опросников (EQ-5D, IPSS, QoL, ВАШ боли). Предполагалось, что аналогичные шкалы будут демонстрировать высокие уровни корреляции (R). Также использовали метод «известных групп»: результаты тестирования пациентов основной группы сравнивались с результатами, полученными при анкетировании респондентов контрольной группы. Проверено наиболее вероятное, «известное» предположение, будто у пациентов с наличием мочеточникового стента баллы, полученные при анкетировании, выше, чем у здоровых людей без стента. При оценке надежности исследовали воспроизводимость и внутреннее постоянство опросника. Воспроизводимость оценивали методом тест–ретест-анализа – пациенты заполняли USSQ дважды через 3 и 4 недели после установки мочеточникового стента. О внутреннем постоянстве судили на основании результатов вычисления коэффициента Кронбаха α; в качестве пороговых определены значения >0,70. Чувствительность USSQ оценивали путем выявления статистически значимых различий при сравнении результатов анкетирования пациентов с установленным мочеточниковым стентом и после его удаления. Статистическую обработку данных осуществляли с использованием программы IBM SPSS Statistics 23. Методы проверки статистических гипотез: критерий Манна–Уитни. Статистически значимыми считали различия при р<0,05.
Результаты
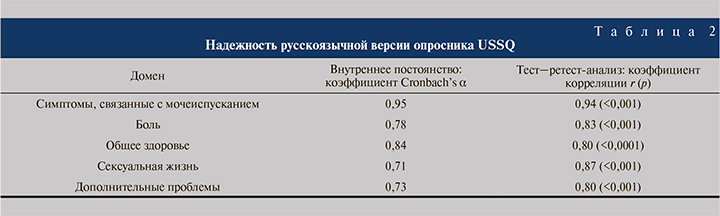
Оценка надежности. Необходимым условием оценки воспроизводимости методом тест–ретест-анализа стало отсутствие изменений в состоянии здоровья пациентов. Коэффициенты корреляции «тест» и «ретест» были довольно высокими и составили 0,80–0,94.
Результат оценки внутреннего постоянства опросника по коэффициенту Кронбаха α варьировался по доменам от 0,95 (симптомы, связанные с мочеиспусканием) до 0,73 (половая жизнь). Таким образом, высокое значение коэффициента Кронбаха α и результаты исследований воспроизводимости опросника подтверждают надежность его русскоязычной версии (табл. 2).
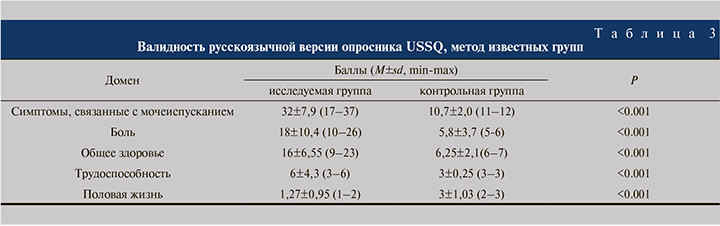
Оценка валидности. Методом «известных групп» подтверждено наиболее вероятное «известное» предположение о том, что у пациентов с наличием мочеточникового стента баллы, полученные при анкетировании, выше, чем у здоровых людей без стента (табл. 3).
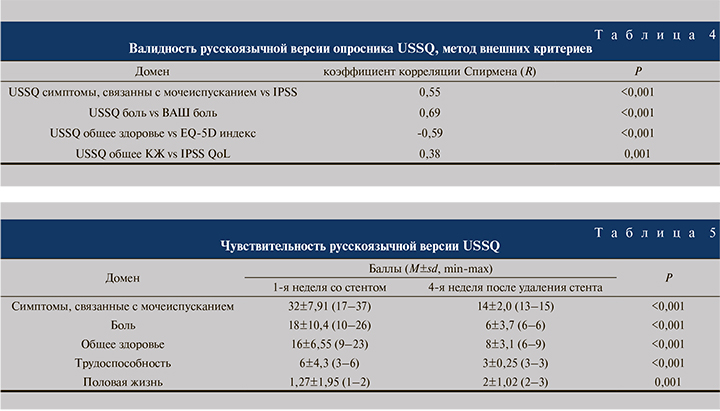
Для оценки критериальной валидности в качестве внешних критериев были выбраны валидные опросники EQ-5D, IPSS, QoL, ВАШ боли. Установлена выраженная корреляция между доменом «симптомы, связанные с мочеиспусканием» и общим баллом IPSS. Также установлена корреляционная связь между доменом «боль» и ВАШ боли, доменом «общее здоровье» и EQ-индексом (табл. 4).
Таким образом, имеются довольно сильные статистически значимые корреляционные связи с основными объективными показателями, т.е. конструкция опросника позволяет достоверно оценивать специфические симптомы и КЖ у пациентов рассматриваемой группы.
Оценка чувствительности. Через 4 нед. после удаления мочеточникового стента оценка симптоматики и КЖ проведена 56 из 70 респондентов. Четырнадцать пациентов на контрольный осмотр, включивший повторное анкетирование, не явились. При анкетировании отмечено статистически значимое снижение баллов по всем доменам русскоязычной версии USSQ (табл. 5).
Таким образом, опросник – это достаточно чувствительный инструмент оценки симптомов и КЖ пациентов с мочеточниковым стентом, может применяться как в клинической практике, так и при проведении исследований. Широкое использование USSQ в клинических исследованиях по оценке КЖ пациентов с мочеточниковым стентом обеспечит большую достоверность результатов и облегчит мета-анализ.
Обсуждение. В настоящее время изменяется подход к оценке результатов оказания медицинской помощи больному. Клиническое выздоровление, подтвержденное лишь данными лабораторного и инструментального обследований, не является полноценным, так как не всегда приводит к нормализации психологического и социального функционирования пациента. Проблема качества жизни пациентов с внутренними стентами носит весьма актуальный характер и до сих пор не решена. На протяжении последних лет опубликовано несколько работ, в которых для изучения частоты возникновения и степени выраженности «стент-ассоциированных» симптомов использовали опросник, предложенный в 1998 г. С. С. Зенковым [1–3], либо анкеты для оценки симптомов нижних мочевыводящих путей (IPSS, QoL, BFLUTS). Все они не были валидированы для пациентов с мочеточниковыми стентами, поэтому не могут достоверно оценивать КЖ и полностью отражать весь спектр «стент-ассоциированных» симптомов, что противоречит основным принципам методологии изучения КЖ в медицине [7]. В настоящее время единственным специальным валидированным инструментом оценки КЖ и симптомов, связанных с мочеточниковым стентом, остается опросник USSQ. Данная анкета получила международное признание и широко применяется в различных клинических исследованиях [20–21].
В ходе проведения оценки психометрических свойств русскоязычной версии USSQ показана высокая надежность, валидность и чувствительность опросника, что говорит о хорошей языковой и культурной адаптации данного инструмента для русскоязычной популяции.
Ввиду многомерности оценки КЖ и «стент-ассоциированных» симптомов, некоторой сложности интерпретации результатов данный инструмент в большей степени подходит для применения в клинических исследованиях, нежели для рутинного использования. Наличие специального валидного инструмента для оценки КЖ и симптомов, связанных с мочеточниковым стентом, позволит достоверней проводить сравнительные исследования различных конфигураций и материалов мочеточниковых стентов, в том числе и в международных исследованиях.
Вывод. После проведения языковой и культурной адаптации опросника USSQ, согласно международным стандартам, с учетом этно-лингвистических особенностей популяции создана русскоязычная версия опросника, эквивалентная оригиналу, характеризующаяся высокими психометрическими свойствами, что позволяет широко применять данный инструмент в клинических исследованиях оценки качества жизни и симптомов, связанных с мочеточниковым стентом.







