Введение. Доброкачественная гиперплазия предстательной железы (ДГПЖ) входит в число наиболее значимых урологических заболеваний [1, 2]. В возрасте до 60 лет ДГПЖ диагностируют более чем у 42% мужчин. К 90 годам заболевание выявляют практически у 90% мужчин. При этом более 30% из них не менее 1 раза в течение жизни подвергаются хирургическому лечению [3–7].
Различные варианты воспалительной реакции являются постоянным компонентом стромальных изменений в предстательной железе (ПЖ) при ДГПЖ. По результатам ряда исследований, посвященных данной проблеме, у 57,2% больных хроническим простатитом (ХП) имелась ДГПЖ, у 38,7% с ДГПЖ – ХП [8]. При гистологическом исследовании у 96,7% больных ДГПЖ обнаруживают морфологические признаки хронического воспаления, которое во многих случаях протекает латентно или асимптомно [9]. При этом вялотекущие воспалительные явления в ПЖ также приводят к фиброзным изменениям, нарушению микроциркуляции, по некоторым данным, и к нарушению аутофагоцитоза в ткани железы, которое также может приводить к гиперплазии [10, 11]. Сопутствующий воспалительный процесс не только играет роль в патогенезе ДГПЖ, но и отражается на клиническом течении заболевания, приводя к увеличению выраженности симптомов нарушенного мочеиспускания [12–14].
В настоящее время препаратами выбора лечения больных данной категории являются α-адреноблокаторы (α-АБ), ингибиторы 5α-редуктазы и их комбинаци [1, 2, 15–18]. С учетом патогенетических процессов, протекающих в ПЖ при ДГПЖ, обоснованным представляется применение препарата Лонгидаза®, обладающего противовоспалительными и иммуномодулирующими свойствами, а также уменьшающего выраженность фиброзных процессов в тканях [19].
Цель исследования: оценка эффективности препарата Лонгидаза® в лечении мужчин с ДГПЖ.
Материалы и методы. В исследование включены 120 пациентов с симптомами нижних мочевыводящих путей (СНМП), обусловленными ДГПЖ. Возраст пациентов составил от 45 до 70 лет.
Критерии включения: суммарный балл IPSS – 8–19, объем остаточной мочи (Vом) до 50 мл, объем ПЖ (Vпж) более 30 см3, уровень простатспецифического антигена (ПСА) до 4 нг/мл, подписанное информированное согласие на терапию препаратом Лонгидаза® и дальнейшее наблюдение (исследование одобрено этическим комитетом ФГБОУ ВО «Воронежский государственный медицинский университет им. Н. Н. Бурденко» МЗ РФ).
Критерии невключения/исключения: конкременты мочевого пузыря и дистального отдела мочеточников, гематурия, отягощенный аллергический анамнез, оперативные пособия на органах малого таза, инфекции мочевыводящих путей в активной фазе, нейрогенная дисфункция мочевого пузыря, врожденные аномалии развития МПС, онкологические и тяжелые сердечно-сосудистые заболевания, сахарный диабет, гипогонадизм.
Пациенты рандомизированы на 2 группы по 60 человек.
В основной группе (ОГ) проводилась терапия препаратом Лонгидаза® в дозировке 3000 МЕ (ректальные суппозитории) по схеме: по 1 суппозиторию через день, 10 введений, далее через 2 дня еще 10 введений (всего 50 дней), после чего назначалась поддерживающая терапия по 1 суппозиторию 1 раз в 7 дней, 4 мес. В качестве фоновой терапии пациенты принимали тамсулозин 0,4 мг 1 раз в день per os в течение всего периода наблюдения.
В группе сравнения (ГС) пациенты получали только тамсулозином 0,4 мг 1 раз в день per os в течение всего периода наблюдения.
Обследование пациентов проходило в ходе 5 визитов.
При обращении пациентов проводился скрининговый визит (день -5–-1), на котором им было предложено участие в исследовании и в случае согласия назначались обследования, необходимые для оценки критериев включения и невключения. Они включали сбор жалоб и анамнеза, заполнение шкалы IPSS и оценки качества жизни QoL, международного индекса эректильной дисфункции IIEF, дневника мочеиспусканий, пальцевое ректальное исследование (ПРИ), урофлоуметрию с определением максимальной скорости потока мочи (Qmax), трансректальное УЗИ ПЖ, УЗИ почек и мочевого пузыря с определением объема ПЖ (Vпж) и объема остаточной мочи (Vом), общеклинические анализы крови и мочи.
На визите 1 (день 1) по результатам проведенных обследований оценивали исходное состояние пациентов, наличие критериев включения/невключения в исследование, а также проводили рандомизацию пациентов в группы и выдачу препарата.
Эффективность лечения оценивали на визитах 2 (день 50±3), 3 (день 106±3) и 4 (день 162±3) по следующим показателям: изменение Vпж, Vом, Qmax, среднего балла по шкалам IPSS, QoL и IIEF по сравнению с исходным уровнем. Кроме того, оценивали комплаентность – приверженность пациентов проводимой терапии (выражали в % больных, соблюдавших рекомендации доктора в периоды между визитами), а также частоту возникновения у пациентов нежелательных явлений, связанных с приемом препарата Лонгидаза®.
Статистическая обработка результатов проводилась с помощью программы MS Exel 11.0 из стандартного пакета MS Office. 2013, а также программного обеспечения IBM SPSS Statistics 21.0. При проверке статистических гипотез применяли методы параметрической (t-test Cтьюдента) статистики. При оценке достоверности выявленных различий между средними значениями выборок рассчитывали параметр р, вероятность справедливости нулевой гипотезы была принята равной 5% (р<0,05).
Результаты. Пациенты в обеих группах полностью соответствовали критериям включения в исследование, критериев исключения ни у кого выявлено не было. Исследуемые группы были однородными, результаты обследований, полученные до лечения, статистически значимо не различались. Исходно в обеих группах выявлено увеличение Vпж, наличие остаточной мочи в мочевой пузыре, СНМП по данным опросника IPSS, нарушения эрекции различной степени выраженности по результатам заполнения опросника IIEF и снижение качества жизни по шкале QoL.
В течение периода лечения комплаентность составила 100%, все пациенты соблюдали назначения врача, случаев отказа от терапии не было. Кроме того, не было отмечено случаев нежелательных явлений, связанных с приемом препарата Лонгидаза®.
Согласно результатам заполнения шкалы IPSS, динамика данного показателя была лучше в группе пациентов, получавших комбинированную терапию (см. таблицу). Исследуемый показатель в данной группе за весь период наблюдения в среднем уменьшился на 5,4 балла, что составило 30,5% от исходного значения. При этом различия между группами были статистически значимыми начиная с Визита 2 до окончания периода наблюдения.
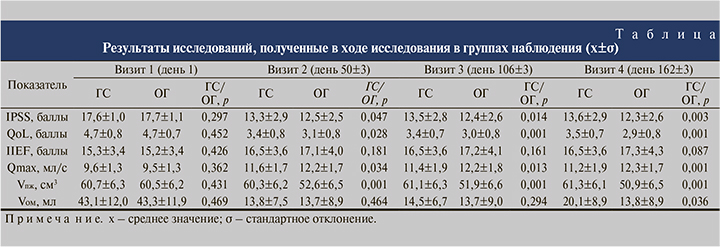
При оценке качества жизни по шкале QoL исходно средний балл в обеих группах составил 4,7, субъективная оценка пациентов варьировалась от неудовлетворительной до очень плохой (см. таблицу). В целом в группе пациентов, получавших Лонгидазу, после начала терапии качество жизни, согласно результатам заполнения опросника QoL, было выше (рис. 1, 2).
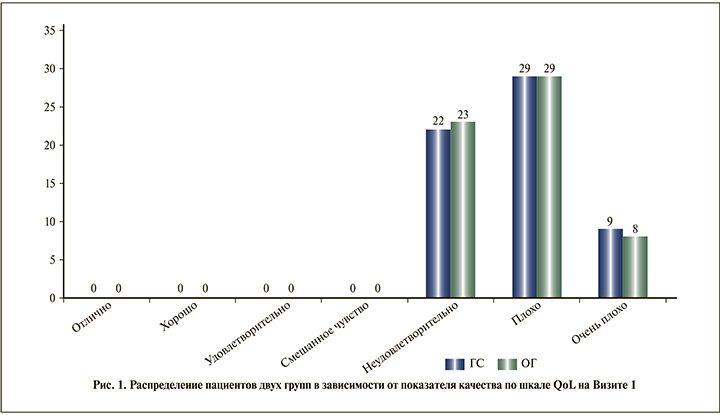

В обеих группах отмечено увеличение среднего балла по шкале IIEF, но, несмотря на более выраженную положительную динамику в ОГ, различия не достигли уровня статистической значимости (p=0,087) (см. таблицу). Однако с учетом выявленной динамики и возрастных особенностей пациентов исследуемых групп нами был проведен анализ отдельных случаев и определено число пациентов с различной степенью тяжести эректильной дисфункции (рис. 3, 4).
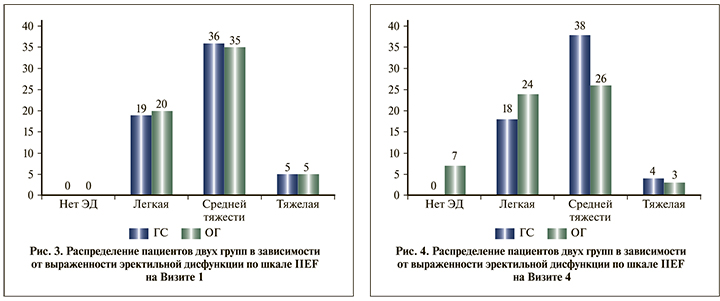
Кроме того, принимая во внимание возрастные особенности пациентов, а также тот факт, что эректильная дисфункция не была причиной обращения пациентов к врачу и дизайн исследования не предусматривал критерии включения и исключения по признаку эректильной дисфункции, при анализе результатов заполнения шкалы IIEF отдельное внимание было уделено общей сексуальной удовлетворенности пациентов (рис. 5).
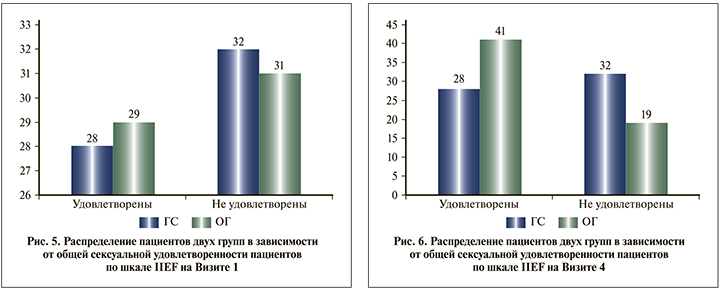
Согласно полученным данным в ГС, субъективная удовлетворенность пациентов собственной половой жизнью не изменилась, тогда как в ОГ число пациентов, удовлетворенных сексуальной жизнью, увеличилось на 12 (41,3%) по сравнению с исходным количеством (рис. 6).
В целом за весь период наблюдения в ГС среднее значение Qmax увеличилось на 1,6 (16,7%), в ОГ – на 2,8 мл/с (29,5%) (см. таблицу).
В течение периода наблюдения в ГС показатель Vпж увеличился в среднем на 0,6 см3, тогда как в группе пациентов, получавших комбинированную терапию с Лонгидазой, уменьшился в среднем на 9,6 см3, что составило 15,9% от исходного объема (см. таблицу). При этом различия между группами по данному показателю начиная с Визита 2 до окончания периода наблюдения были статистически значимыми (p<0,05).
По результатам УЗИ на Визите 2 отмечена выраженная положительная динамика показателя Vом, среднее значение в ГС и ОГ уменьшилось (см. таблицу). Статистически значимых различий между группами не выявлено (p=0,464). На Визитах 3 и 4 средний Vом в группах также был в пределах нормальных значений, однако в ГС показатель к концу периода наблюдения увеличился до 20,1±9,4 см3 и статистически значимо отличался от значения в ОГ (p=0,001), которое существенно не изменилось начиная с Визита 2 до конца периода наблюдения.
Обсуждение. Доброкачественная гиперплазия предстательной железы – полиэтиологичное заболевание, возникающее вследствие разрастания периуретральной железистой зоны ПЖ, приводящего к обструкции нижних мочевыводящих путей [1, 2].
Возникновение и развитие обструктивных и ирритативных симптомов нарушенного мочеиспускания при ДГПЖ определяются двумя составляющими: статическим в результате механического сдавления уретры гиперплазированной тканью ПЖ и динамическим, обусловленным гиперактивностью α-адренорецепторов шейки мочевого пузыря, простатического отдела уретры и ПЖ [15–17].
Динамическая составляющая симптомов заболевания связана с повышенным тонусом α1-адренергических рецепторов ПЖ и шейки мочевого пузыря, что обусловливает сокращение гладкомышечных элементов данной анатомической зоны. Назначение препаратов группы α1-АБ, расслабляющих гладкомышечные элементы ПЖ, приводит к снижению степени обструкции [15].
В патогенезе нарушений мочеиспускания (СНМП) одним из основных факторов является развитие фиброза ПЖ в результате асептического воспаления, которое зачастую протекает латентно или асимптомно [20–23]. Избыточное отложение коллагена и фиброз в ПЖ нарушает функцию уретры у мужчин и приводит к появлению СНМП. Выявлена обратная корреляция результатов урофлоуметрии с фиброзом ПЖ и выраженностью СНМП у больных ДГПЖ. Также было показано, что ткань биоптатов ПЖ у мужчин с умеренными/выраженными СНМП имеет большую механическую ригидность и значительно более высокое содержание коллагена, чем у мужчин с отсутствием или легкими СНМП [24–26].
Кроме того, опубликовано несколько работ, посвященных изучению дефектов протекания воспалительного процесса в тканях ПЖ в виде нарушения аутофагоцитоза: считается, что последние могут быть ответственными за развитие как ДГПЖ, так и рака ПЖ [10, 11]. C. de Nunzio et al. определяли индекс воспаления, связанный с нарушением аутофагоцитоза, в тканях ПЖ, удаленных путем трансуретральной резекции, у 50 пациентов. Критериями активности воспалительного процесса считали уровни экспрессии белков LC3B и р62. Воспалительный индекс был выше у пациентов с низким уровнем LC3B и высоким уровнем p62, которые ассоциировались со снижением процессов аутофагоцитоза. Таким образом, авторы связывают активность воспалительного процесса с повышенным риском прогрессирования ДГПЖ на основании экспрессии данных белков в биоптатах ПЖ [10, 11].
В свете представленных выше данных обоснованным может быть применение препаратов, обладающих противовоспалительными и иммуномодулирующими свойствами, а также уменьшающих выраженность фиброзных процессов в тканях.
В настоящее время на фармацевтическом рынке РФ зарегистрирован препарат Лонгидаза®, суппозитории вагинальные и ректальные на основе масла какао. Он обладает ферментативной (гиалуронидазной) активностью и пролонгированным действием. Пролонгирование действия достигается ковалентным связыванием фермента с физиологически активным высокомолекулярным носителем (сополимер N-оксида 1,4-этиленпиперазина и [N-карбокси-метил]-1,4-этиленпиперазиний бромид). Оно лежит в основе хелатирующего, антирадикального, иммуномодулирующего и противовоспалительного свойств препарата, а также обеспечивает как местное, так и системное действие препарата [24–26].
Несмотря на массу публикаций, посвященных применению Лонгидазы при ХП, данных об эффективности препарата для пациентов с ДГПЖ не так много. Однако представленные в литературе данные подтверждают описанные патогенетические эффекты этого препарата [27–29].
Эффективность препарата Лонгидаза® в коррекции местных иммунометаболических нарушений изучена в исследовании с участием 81 пациента в возрасте от 50 до 70 лет, страдавшего аденомой предстательной железы в сочетании с ХП, в период с 2007 по 2009 г. [20]. По результатам исследования выявлено, что у этих пациентов на местном уровне уже при поступлении в клинику имелись иммунные и оксидантные изменения, выразившиеся повышением содержания большинства исследованных провоспалительных цитокинов (ФНОα, ИЛ-1β, -2, -8, ИФНα), снижением уровня противовоспалительного ИЛ-10, активацией системы комплемента по классическому и альтернативному пути с замедленным повышением уровня ингибиторов системы комплемента, уменьшением уровня sIgA, активацией процессов перекисного окисления липидов, снижением NO-синтетической активности эндотелия капилляров. Включение в лечение свечей Лонгидаза® оказалось эффективным в коррекции лабораторных показателей иммунного и оксидантного статусов.
Клинико-иммунологическая эффективность Лонгидазы для больных ДГПЖ в сочетании с ХП изучена М. Н. Шатохиным и соавт. [28]. Показано, что включение в комплексное лечение изучаемого препарата позволило скорригировать нарушенные показатели содержания иммунофенотипированных лимфоцитов, концентрации цитокинов, компонентов системы комплемента и ее регуляторов, а также функциональной активности нейтрофилов периферической крови.
С. В. Котов и соавт. [29] опубликовали результаты мультицентрового рандомизированного исследования эффективности бовгиалуронидазы азоксимера (Лонгидаза®) у мужчин после трансуретральной резекции ПЖ, в которое были включены 202 пациента [29]. Согласно полученным данным применение Лонгидазы приводит к уменьшению отека простаты, снижает частоту повторной бактериурии, лейкоцитурии, что выражается в тенденции к снижению частоты инфекционных осложнений и дополнительного назначения антибактериальных препаратов, а отсутствие связанных с препаратом побочных явлений за время наблюдения характеризует препарат благоприятным соотношением польза/риск.
Результаты, полученные нами в ходе исследования, в целом согласуются в данными, представленными в литературе [20, 28, 29]. В обеих группах на фоне терапии отмечено уменьшение выраженности СНМП, что свидетельствует об эффективности проведенного лечения. Кроме того, между группами выявлены статистически значимые различия (p<0,05) по всем показателям, кроме среднего балла по шкале IIEF (см. таблицу).
Динамика среднего балла по шкале IPSS характеризовалась резким его снижением в обеих группах, что обусловлено действием α-АБ на динамический компонент инфравезикальной обструкции путем расслабления гладкой мускулатуры нижних мочевыводящих путей. При этом уменьшение показателя на фоне приема Лонгидазы было статистически более значимым. Это может быть связано как с влиянием на латентно текущее воспаление у ряда пациентов в группе, что проявлялось уменьшением выраженности симптомов, вызванных им, так и с уменьшением отека ткани ПЖ и воздействием таким образом на механический компонент обструкции. Дальнейшее наблюдение продемонстрировало незначительную отрицательную динамику в ГС, вероятно обусловленную ростом ПЖ у ряда пациентов в группе. В ОГ, напротив, была зафиксирована положительная динамика на фоне продолжавшейся терапии препаратом Лонгидаза® за счет его антипролиферативного и противовоспалительного эффектов, которые позволили предотвратить нарастание клинической симптоматики. По результатам оценки качества жизни с использованием опросника QoL показатели в ОГ также были статистически значимо лучше, чем в ГС (p<0,05), на протяжении всего периода наблюдения.
Отдельное внимание следует уделить динамике среднего балла по шкале IIEF в группах. Несмотря на отсутствие статистически значимых различий между группами, динамика показателя в ОГ была лучше: если в ГС субъективная удовлетворенность пациентов собственной половой жизнью не изменилась, то в ОГ число пациентов, удовлетворенных сексуальной жизнью, увеличилось на 12 (41,3%).
Эффективность Лонгидазы в терапии эректильной дисфункции, безусловно, требует подтверждения в отдельных исследованиях с соответствующим дизайном и контингентом больных, однако предварительные результаты, полученные в ходе исследования, могут быть обусловлены улучшением микроциркуляции, снижением воспалительной реакции и отека в малом тазу, а также воздействием на психогенные причины эректильной дисфункции на фоне положительной динамики клинического течения ДГПЖ.
При определении Qmax на Визите 2 на фоне приема α-АБ среднее значение показателя в обеих группах увеличилось более чем на 20% и сохранялось примерно на одном уровне на протяжении всего исследования. Тем не менее результаты в ОГ были статистически значимо лучше (p=0,001), а в ГС была зафиксирована незначительная отрицательная динамика. На наш взгляд, полученные результаты обусловлены теми же причинами, что и в случае с оценкой по шкале IPSS.
Наиболее значимые результаты получены при оценке динамики объема ПЖ. После 50 дней наблюдения на Визите 2 в ГС среднее значение показателя Vпж существенно не изменилось. Подобные результаты в данной группе ожидаемы, так как терапия α-АБ не влияет на статический компонент обструкции и на размеры ПЖ. Зафиксированное на последующих визитах увеличение данного показателя также объясняется тем, что α-АБ не позволяют снижать риск прогрессировния гиперплазии ПЖ.
В ОГ на Визите 2 среднее значение Vпж уменьшилось на 7,9 см3, т.е. на 13% относительно исходного размера. Полученный на данном этапе результат, на наш взгляд, связан с уменьшением отека ткани железы за счет устранения вялотекущего латентного воспалительного процесса у ряда пациентов и изменения консистенции секрета ПЖ, способствующего дренированию ацинусов.
На Визитах 3 и 4 также было зафиксировано уменьшение среднего значения Vпж на 0,7 и 1,0 см3 соответственно. Отсутствие роста ПЖ в ОГ в отличие от ГС, с одной стороны, может быть связано со способностью входящей в состав препарата гиалуронидазы подавлять рост и вызывать регресс соединительнотканных образований, с другой – с уменьшением отека на фоне купирования латентного воспалительного процесса и улучшения микроциркуляции в ткани железы, а также, возможно, с сохранением нормальных процессов аутофагоцитоза за счет устранения и профилактики развития воспаления в ткани ПЖ на фоне поддерживающей терапии препаратом.
Среднее значение объема остаточной мочи на Визите 2 на фоне приема α-АБ в обеих группах уменьшилось. Тем не менее на последующих визитах обращает на себя внимание незначительная отрицательная динамика в ГС, при этом различия между группами к моменту окончания исследования достигли уровня статистической значимости (p=0,036). На наш взгляд, причины подобной динамики такие же, как и в случае с -PSS и Qmax.
Таким образом, согласно представленным в литературе данным, ДГПЖ в большинстве случаев сопровождается вялотекущим асептическим воспалительным процессом, который приводит к фиброзным изменениям ткани железы, отеку, гипоксии, нарушению аутофагоцитоза и местного иммунитета, что в свою очередь, с одной стороны, способствует хронизации воспалительного процесса, с другой – к прогрессированию ДГПЖ, увеличению объема ПЖ и нарастанию клинической симптоматики за счет статического компонента.
Применение препарата Лонгидаза® может способствовать уменьшению гиперплазии за счет ферментативной активности, уменьшения выраженности сопутствующего воспаления при ДГПЖ, а также более эффективного действия препаратов фоновой терапии. Гиалуронидаза способна подавлять рост и вызывать регресс соединительнотканных образований, фенотипические особенности которой приобретает строма в области гиперплазии.
Заключение. Таким образом, согласно полученным в ходе данного наблюдательного исследования результатам, препарат Лонгидаза® может эффективно применяться пациентами с ДГПЖ, препятствовать прогрессированию заболевания, способствовать стойкому купированию СНМП и повышению качества жизни пациентов. При этом препарат имеет отличный уровень комплаентности и высокий профиль безопасности. Однако с учетом относительно небольшого количества исследований эффективности и безопасности Лонгидазы при ДГПЖ для точного определения механизмов действия и подтверждения полученных результатов необходимо проведение исследований, в том числе многоцентровых и плацебоконтролируемых. Кроме того, отдельно следует изучить эффективность Лонгидазы при лечении пациентов с нарушениями эрекции.



