Введение. Рак предстательной железы (РПЖ) остается важной проблемой в современной онкологии, занимает 2-е (14,5%) место в структуре онкологической заболеваемости и 3-е (8,1%) – в структуре онкологической летальности мужского населения России [1]. Несмотря на уменьшение доли больных III–IV стадий и рост выявляемости раннего РПЖ с использованием современных методов визуализации [2, 3], по темпам прироста смертности среди мужчин за последние 10 лет данная онкопатология занимает 1-е (+13,9%) место на фоне значительного снижения стандартизованного показателя смертности от всех злокачественных новообразований (-14,5%).
Выбор тактики лечения больных РПЖ без выявленных отдаленных метастазов зависит прежде всего от риска прогрессирования опухолевого процесса [4–6]. Известно, что клиническое течение заболевания значительно варьируется от высокоагрессивной формы до индолентной. Наиболее широкое практическое применение получила классификация риска D’Amico с выделением трех групп – низкого, промежуточного или высокого рисков [7]. Российские и международные профессиональные сообщества рекомендуют использование данной классификации в различных модификациях, которые могут различаться по количеству выделяемых групп риска (от 3 до 7), но используют одни и те же клинико-морфологические характеристики заболевания: уровень простатического специфического антигена (ПСА) на момент выявления РПЖ, степень дифференцировки опухоли по шкале Глисона (индекс Глисона) и клиническую стадию [4–6, 8]. Валидация модели риска D’Amico подтвердила ее прогностическую значимость для выживаемости без рецидива больных, получивших радикальную лучевую терапию (ЛТ) или перенесших радикальную простатэктомию (РПЭ) [7, 9, 10].
Существенный недостаток классификации D’Amico связан с выявленной гетерогенностью результатов лечения больных в каждой из групп риска: например, выделена подгруппа «благоприятного» высокого риска [11], стратифицированы подгруппы очень высокого риска, благоприятного и неблагоприятного промежуточного риска [12], обнаружена когорта больных со скрытым неблагоприятным прогнозом в группе низкого риска [13]. Для оптимизации модели риска может быть использовано два пути: во-первых, дальнейшее увеличение групп риска (например, до 7 групп в рекомендациях NCCN [4]) или добавление независимых маркеров неблагоприятного прогноза. В настоящее время выявлен широкий спектр факторов, преимущественно патоморфологических (наличие периневральной или лимфоваскулярной инвазии, криброзных структур в опухоли), но также иммуногистохимических (экспрессия Ki-67 в опухоли) и молекулярно-генетических (экспрессия каликреина 3, мутации в генах BRCA1/2 и CHEK2), ассоциированных с ухудшением отдаленных результатов радикального лечения РПЖ (табл. 1) [14–23].

Новым потенциально перспективным маркером может быть экспрессия лиганда белка программируемой клеточной гибели (programmed cell death protein ligand, PD-L1) в опухоли, ключевого компонента сигнального пути контрольных точек иммунного ответа, стимулирующего апоптоз антигенспецифичных Т-лимфоцитов в лимфатических узлах и одновременно подавляющего апоптоз регуляторных супрессорных Т-лимфоцитов (Tregs). В нормальных условиях сигнальный путь PD/PD-L1 регулирует степень выраженности и длительность иммунного ответа, защиту от аутоиммунных реакций и повреждения собственных тканей. Экспрессия PD-L1 в опухоли ассоциирована с неблагоприятным клиническим течением и снижением выживаемости больных колоректальным раком [24], раком молочной железы [25], поджелудочной железы [26] и других злокачественных новообразований [27 – 31].
Цель исследования: оценить прогностическое влияние PD-L1-статуса в опухолевых клетках на отдаленные результаты радикального лечения больных РПЖ.
Материалы и методы. В ретроспективный анализ были включены данные 45 пациентов с гистологически верифицированной аденокарциномой предстательной железы, находившихся под наблюдением в НМИЦ онкологии им. Н. Н. Блохина после радикального лечения: радикальной простатэктомии (РПЭ; n=39), лучевой терапии (ЛТ; n=6). Значительную (71,1%) долю составили больные, у которых в момент наблюдения выявлено прогрессирование опухолевого процесса с развитием отдаленных метастазов.
У всех пациентов определяли экспрессию PD-L1 в опухолевых клетках с использованием метода иммуногистохимического исследования срезов парафиновых блоков, полученных под контролем патоморфолога с применением моноклонального антитела Anti-PD-L1 antibody (28-8) (ab 205921) на иммуностейнере «Ventana BenchMark GX». За экспрессию PD-L1(+) принимали уровень экспрессии PD-L1 ≥1% в опухолевых клетках; за гиперэкспрессию PD-L1hyper(+) – ≥5%.
Статистическую обработку материалов исследования проводили с помощью пакета прикладных программ IBM SPSS Statistics версии 22 (IBM Corp., США). Для определения различий между качественными показателями пациентов различных групп применяли точный критерий Фишера. Метод параметрической статистики (t-критерий Стьюдента) использовали для оценки различий в группах при нормальном распределении числовых данных, в отсутствие нормального распределения данных использовали методы непараметрической статистики (U-тест Манна–Уитни).
Выживаемость без биохимического рецидива, безметастатическую и опухолевоспецифическую выживаемость больных оценивали по методу Каплана–Майера, статистические различия между группами – с помощью log-rank-теста. Для оценки потенциального влияния различных факторов риска на каждый вид выживаемости проводили однофакторный анализ с использованием непараметрической модели пропорциональных рисков Кокса. В многофакторную модель были включены факторы риска с наиболее значимым влиянием на выживаемость (p<0,2). Статистически значимыми считали различия при р<0,05 (95% уровень значимости).
Результаты. Экспрессия и гиперэкспрессия PD-L1 в опухолевых клетках выявлены в 8 (17,8%) и 6 (13,3%) случаях соответственно. В зависимости от результатов иммуногистохимического анализа пациенты были распределены на 3 группы: без выявленной экспрессии PD-L1(–); с выявленной экспрессией PD-L1(+) и пациенты с выявленной гиперэкспрессией PD-L1hyper(+).
Медиана возраста на момент выявления РПЖ в группе пациентов PD-L1(+) составила 56,5 лет и была ниже, чем в группе PD-L1(-), – 62 года (p=0,094). Уровень ПСА на момент выявления заболевания, распределение по стадиям T и N, гистологической градации опухоли в этих группах статистически значимо не различались. Пациенты с PD-L1hyper(+) статистически значимо не различались по вышеуказанным клиническим характеристикам (табл. 2).
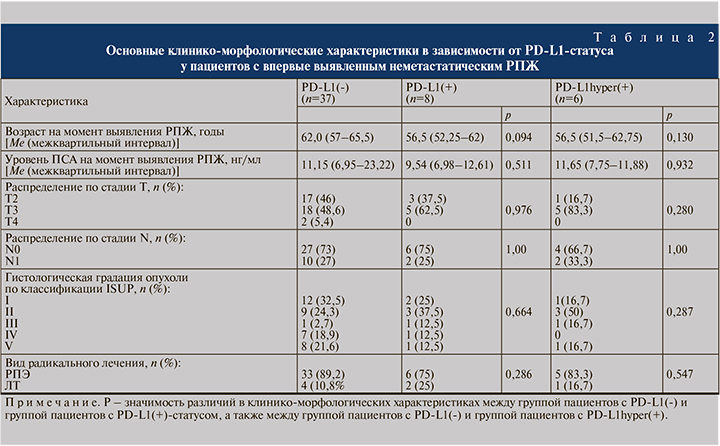
Все три группы оказались сбалансированными по прогностически неблагоприятным патоморфологическим характеристикам. Низкодифференцированные формы опухоли чаще встречались при PD-L1(-) (40,5%), но без достоверных различий по сравнению с PD-L1(+) (25%, p=0,69) и PD-L1hyper(+) (16,7%, p=0,386). В группе PD-L1(-) чаще (75,7%), чем в группе PD-L1(+) (62,5%), встречалась периневральная инвазия опухоли (р=0,661). Напротив, при PD-L1(+) несколько чаще выявляли криброзные структуры и лимфоваскулярную инвазию опухоли, чем при PD-L1(-) (25 против 13,5%, р=0,59, и 30,5 против 42,9%, р=0,665 соответственно) и PD-L1hyper(+) (16,7%, р=1,00; и 50%, р=0,383).
Выживаемость без биохимического рецидива после радикального лечения РПЖ
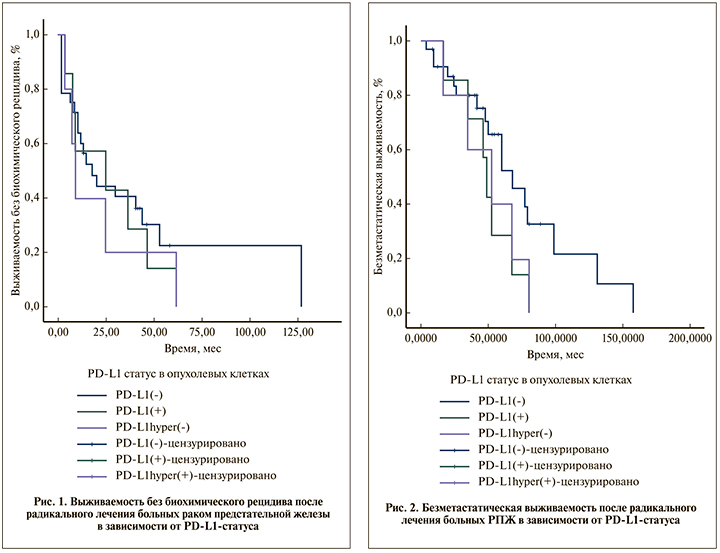
Всего в анализ выживаемости без биохимического рецидива были включены данные 35 больных РПЖ. Критерии исключения пациентов: наличие адъювантной лучевой или гормональной терапии, надир послеоперационного ПСА ≥0,2 нг/мл. При медиане наблюдения 57,9 мес. биохимический рецидив выявлен у 20 (71,4%) пациентов PD-L1(-), у всех 7 (100%) пациентов PD-L1(+) и 5 (100%) пациентов PD-L1hyper(+).
Медиана выживаемости без биохимического рецидива больных во всех 3 группах статистически не различалась и составила 17,77 мес. (95% ДИ – 5,86–29,68) в группе PD-L1(-), 24,85 ме.с (95% ДИ – 0,00–65,49, р=0,575) в группе PD-L1(+) и 9,02 мес. (95% – 4,79–13,24, p=0,402) в группе PD-L1hyper(+). График выживаемости без биохимического рецидива после радикального лечения РПЖ в зависимости от PD-L1-статуса в опухолевых клетках представлен на рис. 1.
Безметастатическая выживаемость после радикального лечения РПЖ
Всего в анализ безметастатической выживаемости включены данные 39 больных РПЖ. Критерии исключения: проведение адъювантной гормональной терапии, надир послеоперационного ПСА ≥0,2 нг/мл. При медиане наблюдения 57,9 мес. метастатическое прогрессирование выявлено у 17 (53,1%) пациентов PD-L1(-), у всех 7 (100%) пациентов PD-L1(+) и 5 (100%) пациентов PD-L1hyper(+).
Медиана безметастатической выживаемости больных в группе PD-L1(+) составила 48,918 мес. (95% ДИ 42,523–55,313) и была ниже, чем в группе PD-L1(-) – 68,033 мес (95% ДИ – 48,242–87,824), различие имело тенденцию к статистической значимости (p=0,090, log-rank-тест). Медиана безметастатической выживаемости больных в группе PD-L1hyper(+) составила 52,197 мес. (95% ДИ – 15,098–89,295) и достоверно не отличалась от таковой группы PD-L1(-) (p=0,181, log-rank-тест). 12-месячная безметастатическая выживаемость после радикального лечения при статусе PD-L1(-) была 90,4±5,3%, при статусе PD-L1(+) и PD-L1hyper(+) – 100%; 5-летняя безметастатическая выживаемость – 65,6±9,7, 28,8±16,1 и 40,0±21,9% соответственно (рис. 2).
С помощью однофакторного анализа Кокса обнаружена тенденция прогностической значимости экспрессии (ОР – 2,161, 95% ДИ – 0,868–5,380, р=0,098) и гиперэкспрессии PD-L1 в опухолевых клетках (ОР – 1,984, 95% ДИ – 0,712–5,524, р=0,190) в отношении безметастатической выживаемости больных после радикального лечения РПЖ (табл. 3). По результатам многофакторного регрессионного анализа Кокса подтверждено независимое прогностическое влияние экспрессии (ОР – 3,461; 95% ДИ – 1,171–10,228; р=0,025) и гиперэкспрессии PD-L1 в опухолевых клетках (ОР – 3,916; 95% ДИ – 1,129–13,591; р=0,032, табл. 4).
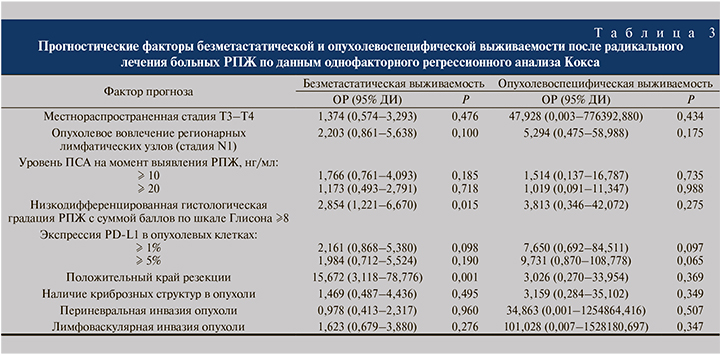
Другими независимыми факторами неблагоприятного прогноза безметастатической выживаемости были положительный край резекции и низкодифференцированная гистологическая форма опухоли.

Опухолевоспецифическая выживаемость после радикального лечения РПЖ
В анализ включены данные всех 45 больных РПЖ. При медиане наблюдения 55,30 мес. летальные исходы от РПЖ выявлены у 2 (5,4%) пациентов группы PD-L1(-), у 2 (25%) – PD-L1(+) и у 2 (33,3%) – PD-L1hyper(+).
Опухолевоспецифическая выживаемость больных группы PD-L1(-) оказалась статистически значимо выше, чем в группах PD-L1(+) (p=0,05) и PD-L1hyper(+) (p=0,024; рис. 3).
Пятилетняя опухолевоспецифическая выживаемость больных после радикального лечения при статусе PD-L1(-) составила 97,1±2,9%, при PD-L1(+) – 87,5±11,7%, при PD-L1hyper(+) – 83,3±15,2%. Единственными факторами с тенденцией значимости для прогноза опухолевоспецифической выживаемости при анализе Кокса оказались экспрессия (ОР – 7,65; 95% ДИ – 0,69–84,51; р=0,097) и гиперэкспрессия PD-L1 в опухолевых клетках (ОР – 9,73; 95% ДИ – 0,87–108,78; р=0,065, табл. 3).
Обсуждение. Результаты нашего исследования показали высокую (17,8%) частоту экспрессии PD-L1 в опухолевых клетках больных первично-неметастатическим РПЖ. Широкая вариабельность экспрессии PD-L1 в первичной опухоли РПЖ, по данным других исследований (от 8 до 92%) [32 – 35], связана с рядом причин: трудностями стандартизации иммунногистохимического анализа, когда результаты зависят от выбора техники подготовки образцов, используемых антител, различающихся по своей специфичности и аффинности к разным эпитопам исследуемых белков, а также субъективными критериями интерпретации полученных данных. Важно отметить, что повышенная активность PD-L1 в опухоли редко связана с активирующими молекулярными перестройками гена PD-L1 [36] и почти всегда обусловлена адаптацией к изменениям иммунного микроокружения опухоли [34, 37, 38].
Наше исследование выявило отсутствие достоверной ассоциации экспрессии PD-L1 в опухолевых клетках РПЖ и рутинно применяемых морфологических факторов неблагоприятного прогноза, что соответствует данным других исследований [33, 39, 40]. Тем не менее в нашем исследовании отдаленные результаты радикального лечения РПЖ оказались хуже при PD-L1(+)-статусе: отмечено снижение безметастатической и опухолевоспецифической выживаемости пациентов (p=0,090 и р=0,05 соответственно) по сравнению с PD-L1(-). В многофакторном анализе Кокса наличие экспрессии PD-L1 в опухолевых клетках служит независимым фактором неблагоприятного прогноза безметастатической выживаемости (ОР – 3,46; 95% ДИ – 1,17–10,23; р=0,025) и опухолево-специфической выживаемости (ОР – 7,65; 95% ДИ – 0,69–84,51; р=0,097). Аналогичные выводы о независимом неблагоприятном прогностическом влиянии экспрессии PD-L1 получены в нескольких крупных многоцентровых исследованиях. Так, H. Gevensleben и et al. обнаружили неблагоприятное влияние на риск биохимического рецидива после РПЭ (ОР – 2,37; 95% ДИ – 1,32–4,25; р=0,004) [35], N. Ness et al. – ассоциацию со снижением выживаемости без клинического прогрессирования после радикального лечения (ОР – 2,48; 95% – 1,12–5,48; р=0,025) и тенденцию влияния на выживаемость без биохимического рецидива (ОР – 1,34; 95% ДИ – 0,97–1,85; р=0,078) [40]. Механизмы неблагоприятного влияния экспрессии PD-L1 в опухоли обусловлены развитием иммунной толерантности к Т-лимфоцитам, активацией апоптоза с истощением пула эффекторных Т-лимфоцитов и усилением иммуносупрессивной функции Tregs, что позволяет опухоли ускользнуть от иммунного ответа и способствует прогрессированию опухолевого процесса. Кроме того, возможны и дополнительные специфические для РПЖ механизмы, так как обнаружена корреляция между экспрессией PD-L1 и экспрессией андрогенновых рецепторов (р<0,001), пролиферацией Ki-67 (p<0,001) [35].
Наше исследование имеет ограничения, обусловленные ретроспективным набором пациентов, малой выборкой и данными одного центра. Тем не менее полученные результаты позволяют рассматривать экспрессию PD-L1 в качестве биомаркера для селекции больных РПЖ с высоким риском прогрессирования заболевания, которым может быть противопоказано активное наблюдение и возможны преимущества от выбора комбинированных подходов к лечению.
Заключение. Результаты нашего исследования и данные публикаций показывают, что наличие экспрессии PD-L1 в опухолевых клетках ассоциированы с неблагоприятным прогнозом клинического течения РПЖ, в частности снижением безметастатической и опухолевоспецифической выживаемости больных после радикального лечения. Механизм неблагоприятного влияния экспрессии PD-L1 в опухоли связан с ключевой ролью данного белка в контроле иммунного ответа. С учетом отсутствия связи экспрессии PD-L1 в опухоли с рутинными клинико-патоморфологическими характеристиками заболевания представляется целесообразным включение экспрессии PD-L1 в действующие номограммы модели риска РПЖ. Полученные результаты могут свидетельствовать о потенциальной целесообразности развития персонализированных подходов к лечению РПЖ, в том числе с использованием лечения с направленным воздействием на сигнальные пути PD-L1/PD-1 в опухолевых клетках.



