Introduction. There are a variety of symptoms associated with an overactive bladder (OAB), which include urgency, frequency, and nocturia, with or without urge urinary incontinence (UUI)[1]. OAB is also associated with decreased quality of life and a high economic cost to society [2].
International Continence Society (ICS) estimates that 12.8% of women and 10.8% of men suffer from OAB; the prevalence of frequency, urgency, and urge incontinence (UI) rises with age [3, 4]. Men’s UUI was significantly lower than women’s. There is a 10–42% prevalence in India, with a progressive increase in prevalence from the third to seventh decade of life (5.6%, 14.2%, 27.3%, 34.3%, and 39%, respectively) [5].
Behavioral and self-control training and other OAB treatment methods are regarded as first-line options for reducing urine incontinence in patients. Antimuscarinics or β3 adrenoceptor agonists are popular treatments for OAB if behavioral changes fail to alleviate symptoms [6]. Some of India’s most commonly prescribed OAB medications among antimuscarinics are solifenacin, oxybutynin, tolterodine, darifenacin, trospium and mirabegron and among anti-adrenoceptor agonists, mirabegron is most common (Table 1) [7].
Antimuscarinics prevent the contraction of the smooth muscle wall around the bladder. Stimulation of the acetylcholine muscarinic M3 receptors in the detrusor muscle wall usually results in micturition. Solifenacin and darifenacin are muscarinic receptor antagonists that only affect the M3 receptor. Oxybutynin and tolterodine are non-selective antimuscarinics that affect all muscarinic receptors, which causes dry mouth [9]. As a beta-3 agonist, mirabegron relaxes the detrusor muscles and enhances bladder storage capacity without affecting voiding contractions. As a result, mirabegron can assist in alleviating the symptoms of OAB [10]. According to a report by the Urological Society of India, OAB is most commonly treated with antimuscarinic or beta-3 adrenoceptor agonists. Most patients with stress urine incontinence turn to the serotonin noradrenaline reuptake inhibitor duloxetine [5, 11].
The objective of the present study is to assess the prescribing practices for OAB pharmacotherapy especially antimuscarinic and beta-3 adrenoceptor agonists, based on the prescription trend analysis across different specialties of India. Moreover, we expect that the accumulation of data from this study will eventually lead to evidence-based medicine that can lead to more advanced OAB treatments irrespective of medical specialties.
Materials and Methods. Data source and setting. From August 2016 to August 2021, we have been using IQVIA Medical Audit Data (formerly IMS Health) to track the urological preparation (G04B) prescription rates for primary care physicians in India who work in the private sector [12]. In more than 100 countries, IQVIA, a non-profit organization, collects market intelligence and disseminates it. Medical audit data monitor prescriptions written by allopathic doctors in private practices. Data were gathered from a random sample of 4600 healthcare practitioners from 23 metropolitan areas of India (over 1 million population), 128 Class 1 cities (population over 100,000), and 1A cities (population fewer than 100,000). A national sample of prescriptions written by doctors in cities with populations greater than one million was drawn from the original data [13].
The data use the European Pharmaceutical Market Research Association’s (EphMRA) anatomical therapeutic classification (ATC), not the World Health Organization’s (WHO) ATC classification. The diagnoses reported on prescriptions are also not coded for the International Classification of Diseases and Related Health Problems (ICD-10). In addition, because the data does not include prescriptions written in the public sector, our analysis only includes outpatient prescription samples from that sector. Last but not least, IQVIA makes the data available to us in aggregate form, processed, and extrapolated to reflect national prescription practices.
Outcome measure. Study focused on the number of antimuscarinic and mirabegron prescribed each year as a primary outcome measure. In addition, study estimated and reported prescriptions by specialty and molecule. For each molecule, number prescribers or their specialties were evaluated.
Statistical analysis. Study used the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, to code prescription diagnosis data from an IQVIA medical audit (ICD-10 classification; version: 2016) [13]. Anatomic Therapeutic Chemical (ATC) classification of antimuscarinics and beta-3 adrenoceptor agonists recommended for the related diagnosis have been coded to the 3rd level of WHOCC’s proposed drug statistics methodology (ATC index-2016) [14]. By searching an online index using keywords in the diagnosis given in the medical audit data, taken directly from the prescription, the diagnosis was assigned an ICD 10 code.
The consumption of Urological preparation (ATC code: G04B), especially antimuscarinics and beta-3 adrenoceptor agonists, as defined in the annual prescription. The medicines prescribed were categorized into these subgroups (ATC codes): solifenacin (G04B0B), darifenacin (G04B07), tolterodine (G04B03), oxybutynin (G04B06), trospium (G04B0A) & mirabegron (G04B0H). Several specialists (Urologist, Nephrologist, Gynaecologist, General Surgeon, Consultant physician) prescribing the above molecules were measured as a prescriber. Also checked the overlap pattern among key molecule’s prescription at urologist’s level. We used software Microsoft Excel 2013 to perform statistical analysis.
Ethics considerations. The data used had no identifiers for the patients. So current study does not require Ethical Committee approval.
Results. Prescription and prescriber data of various antimuscarinics and beta-3 adrenoceptor agonists were analyzed for 6 MAT periods starting from MAT Aug’16 to MAT Aug’21.
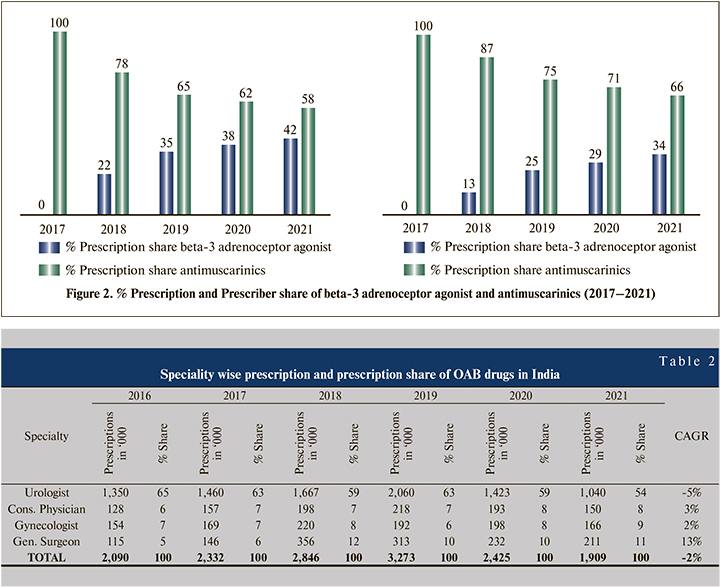
In 2016, for OAB treatment, 84% of prescriptions were generated by the top 4 specialties, i.e., urologist, consultant physician, gynecologist & general surgeon, which reduced to 82% in 2021 with a compound annual growth rate (CAGR) of -2%. In MAT 2021, the urologist is the leading specialty prescribing OAB drugs, with a 54% prescription share. General surgeon contributes 11%, while gynecologist and consultant physician has a 9% & 8% prescription share, respectively (Table 2).
In the last 6 MAT periods, the urologist’s prescription share reduced from 65% to 54%, whereas the general surgeon’s prescription share increased from 5% to 11%.
In 2016, 38% of urologists were contributed 65% of prescriptions in the OAB drug market, which reduces to 33% and 54% in 2021, prescriber contribution and prescription contribution, respectively. At consultant physicians, prescriber contribution increases from 11% to 17%. Still, at the same time, prescription contribution does not grow at the same pace (6% to 8%), mainly because of low prescription per doctor per month (PDM). 8% of surgeons contributed around 5% prescriptions in 2016, which increased to 11% in 2021 due to a positive change in the number of prescriptions per doctor (Figure 1).

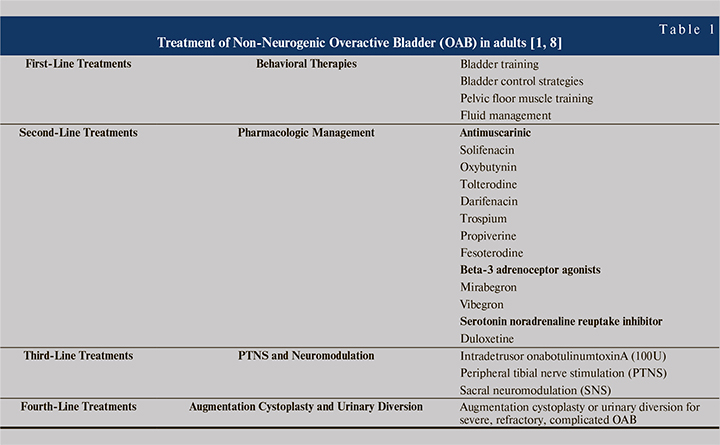
Antimuscarinics and beta-3 adrenoceptor agonists are the preferred drugs for the medical management of overactive bladder in India. Prescription share for antimuscarinics were 100% in 2017 & 58% in 2021 whereas for mirabegron, it was 0% in 2017 & 42% in 2021. Prescriber share data also reflect a similar trend, for antimuscarinics were 100% in 2017 & 66% in 2021 whereas, for mirabegron, it was 0% in 2017 & 34% in 2021 (Figure 2).
Before the launch of Mirabegron in the Indian market, solifenacin was the most preferred drug for the treatment of OAB, with a prescription share of up to 37% in 2017. Mirabegron was approved in India by Central Drugs Standard Control Organisation (CDSCO) in June 2017 & marketed by Nov 2017 15. Antimuscarinics lost their prescription share to mirabegron, whereas, mirabegron gained 42% prescription share with a CAGR of 8% in the OAB market (Table 3).
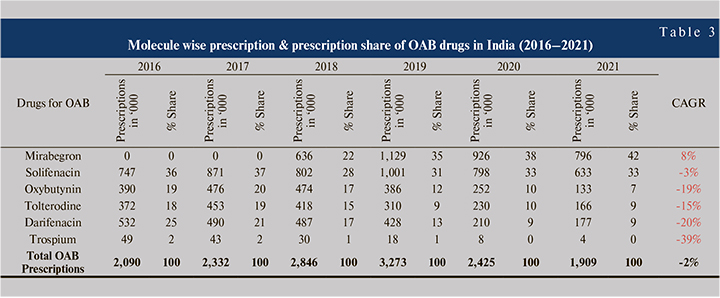
Solifenacin prescription share reduced from 36% to 33%, with a CAGR of -3% during 2016–2021. Major loss in prescription are from oxybutynin (19% - 7%), tolterodine (18% - 9%), and darifenacin (25% - 9%). MAT 2020 period experienced COVID-19 impact in terms of loss in prescription, applicable to all the molecules. Greater relative prescription loss at antimuscarinics than mirabegron indicates the change in prescribing habits or molecular shift in the overactive bladder market.
Urologists were the major specialty prescribing overactive bladder drugs & solifenacin was the most preferred molecule (38% prescription share) by the urologist in India before the launch of mirabegron. Tolterodine was the second preferred drug (29% prescription share) for OAB by urologists in 2016, but in 2021, prescription share reduced to 12%. Darifenacin also lost the prescription share from 18% to 6% (Figure 3).
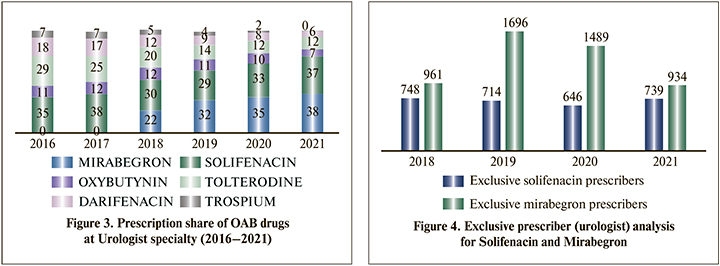
Solifenacin and mirabegron are the most prescribing molecules across the specialties and at urologists. Exclusive prescriber (urologist) analysis has worked out to understand the molecular shift at the prescriber level (Figure 4).
Before the launch of mirabegron, solifenacin dominates the OAB market. But after the launch of mirabegron, a sharp fall was observed in solifenacin prescribers and a significant gain in mirabegron prescribers. In recent years, few early adopters of mirabegron shifted again to solifenacin; also, many urologists prescribe both molecules. The downturn in solifenacin exclusive prescribers and up to gain in mirabegron exclusive prescribers show the molecular shift at the urologist level from solifenacin to mirabegron.
Discussion. The urology drug market contributes almost 1.6% to the Indian pharmaceutical market. Drugs for OAB contribute 7.3% of the total urology market. Although the prevalence of OAB in India ranges from 10–42% [16], the market size is minuscule, just 0.1% concerning the Indian pharmaceutical market [16].
Among Indian urologists, solifenacin (46.7%) is the most commonly prescribed drug for UUI, according to a survey conducted by the Urological Society of India (November 2017). In the two months following the launch of mirabegron in India, a 4.5% preference was recorded, and its use patterns may change in the future [11].
The efficacy and tolerability data unveil the reason behind shifting the molecular preference of Indian clinicians within the antimuscarinics or antimuscarinics to mirabegron. Many studies have compared the efficacy and tolerability of antimuscarinics vs. mirabegron and solifenacin vs. mirabegron. The majority of studies concluded antimuscarinics/solifenacin are comparable with mirabegron in effectiveness, but mirabegron scores more on tolerability and thereby therapy adherence [17–21].
Solifenacin reported the highest persistence rate, 58% and 35%, over 3 months and 12 month period, respectively, versus other antimuscarinics [22]. Solifenacin is a combination of comparable efficacy, lesser side-effects and high therapy adherence, which may be a reason for the clinician’s high preference towards solifenacin over other antimuscarinics.
Mirabegron is an oral adrenoceptor agonist that provides an alternative to antimuscarinics for patients with OAB, as phase III trials of mirabegron versus placebo found significant improvements in key efficacy measures (e.g., urinary incontinence and frequency of urination) [17]. Researchers compared antimuscarinics and mirabegron 50mg in patients with OAB by conducting a systematic literature review and network meta-analysis based on peer-reviewed articles published between 2000 and 2013. Mirabegron 50 mg was as effective as antimuscarinics (excluding solifenacin 10 mg) for urinary frequency, incontinence, and UUI episodes, according to 44 RCTs involving 27,309 patients [23]. Mirabegron shows lesser AEs than antimuscarinics, which has been cited as a major reason for better persistence and therapy adherence rate. 1-year persistence was 12%–25% for antimuscarinic treatment and 32%–38% for mirabegron. Proposed reasons for the lower persistence rate for antimuscarinics include higher bothersome AEs, particularly dry-mouth, and unmet expectations of antimuscarinic treatment [18].
Better symptomatic relief with patient satisfaction is the primary objective behind any medical treatment. Antimuscarinic fulfils the first requirement and provides better symptom relief, but patient satisfaction is less due to side effects. Patients switching from antimuscarinics to mirabegron for OAB have better outcomes if their baseline OAB symptom scores are higher, such as the OAB-SS and IPSS-S [24]. According to a retrospective cohort study, Mirabegron may reduce the antimuscarinic drug’s dosage and thus improve NDO treatment’s long-term efficacy [25]. High therapy adherence, comparable efficacy, and lower side effects could be why clinicians shift their preference from solifenacin to mirabegron.
Recently CDSCO has approved a fixed-dose combination of solifenacin and mirabegron [26]. The randomized, multicentre Synergy trial studied the long-term safety and efficacy of the combination of mirabegron and solifenacin in patients with overactive bladder compared to monotherapy. The study concluded that the treatment with a combination of solifenacin 5mg and mirabegron 50mg over one year in patients with OAB symptoms was well tolerated and had improved symptoms [27]. Allison et al. claimed that adding mirabegron to antimuscarinic could significantly improve OAB symptoms; adding mirabegron to existing treatments could prevent higher doses of antimuscarinics [28]. In the Indian market, the mirabegron/solifenacin combi pack was introduced in late 2019, whereas the fixed-dose combination was launched in July 2021. Currently, 5 companies are marketing such a combination in the Indian market.
The cost of therapy is another factor affecting therapy adherence for long-term treatment in chronic conditions like OAB. Treatment with mirabegron is more cost-effective than antimuscarinics, leading to higher therapy adherence in cost-conscious patients [29].
OAB is a common disease that causes serious problems such as UTIs, skin infections, bladder stones, falling/fractures in the elderly, sleep disturbances, adverse effects on quality of life, and depression [30]. OAB is often accompanied by chronic diseases such as hypertension and diabetes. It is common for elderly incontinence patients to be managed by a non-urologist, especially in rural areas [31]. Our research demonstrates that non-urologists prescribe older generation antimuscarinics like oxybutynin and darifenacin (oxybutynin prescription share at MAT Aug’21, 15% at consultant physician, 7% at general surgeon & 3% at gynecologist vs. 3% at urologist; darifenacin prescription share at MAT Aug’21, 34% at gynecologist vs. 3% at urologist); additionally, there is a lower prescription rate of mirabegron, a relatively newer class of OAB drug (mirabegron prescription share at MAT Aug’21, 31% at consultant physician, and 37% at gynecologist vs. 49% at urologist). The symptoms of OAB are more likely to be severe in elderly patients, and management by urologists is even more crucial. By analyzing the prescription patterns in this study, we found that the expertise of non-urologists concerning OAB is poor, and prescriptions are outdated considering the availability of more tailored, state-of-the-art medications that could be used in the management of OAB. It is expected that the number of drugs to be used due to various diseases and the OAB drugs used will also be increased. Monitoring and management issues related to the treatment of OAB are fundamentally social problems as the frequency and number of drug exposures will increase along with the side effects of the drug itself and side effects caused by drug interactions. Both urologists and non-urologists can manage OAB, but if a non-urologic expert tries to treat OAB alongside a chronic disease, the management should be based on proper OAB treatment guidelines and prescriptions that should be verified scientific value, merit, and defects of selected medication regimens. Improved education and qualitative management are required in the treatment of OAB, especially in primary physicians, regardless of their specialties. Furthermore, there should be more awareness to improve the quality of care for OAB patients. If the treatment procedure, such as drug selection, medication selection, and medication adherence, is essential, it is advisable to consult with a urologist.
Limitation. Our study is not deprived of limitations. First, the database did not offer detailed, patient-level data on OAB management; only limited OAB data could be included in this study, representing a source of bias. Second, the database didn’t provide detailed, patient-level clinical data regarding OAB, like prostate volume or uroflowmetry parameters. The patient may be taking antimuscarinics or mirabegron for storage lower urinary tract symptoms or other bladder/prostate complications, which was not defined and all prescriptions claimed broadly for OAB. Third, in this study, we could not reflect longitudinal data regarding treatment disruptions or poor treatment due to poor drug adherence. Fourth, IQVIA data were extrapolated to a population of Indian physicians using inverse proportional weight. In doing this, it is assumed that the stable panel generally represents other practices, pharmacies, and hospitals for which IQVIA did not have reliable data. Minor difference in the stable panel creates significant differences in final data output. Fifth, the current study only evaluates prescriptions and prescribers trends of two major classes of drugs, i.e., antimuscarinics and beta-3 adrenoceptor agonists used for the treatments of OAB. No other pharmacotherapy has been evaluated. Despite limitations, our study comprehensively evaluated the change in practicing trends of OAB management.
Conclusion. Urology remained a top prescribing specialty for OAB drugs, although prescription share increased at surgeon and consultant physician specialty. OAB medicines prescriptions by urologists are shifting from leading antimuscarinic solifenacin to beta-agonist mirabegron. Other non-urologist specialties like consultant physicians, general surgeons, and gynecologists continue to prescribe antimuscarinics as a preferred drug for OAB. Antimuscarinics’ prescription share shows a downtrend due to the high incidence of side effects and lowers therapy adherence compared with mirabegron. Among Indian specialists, mirabegron emerges as a promising treatment option for overactive bladder. Recently approved fixed-dose combination of antimuscarinic, solifenacin and beta-3 adrenoceptor agonist, mirabegron is the newer approach in OAB management in India. Data from this study will ultimately lead to the OAB medication preference by the specialist that could lead to improved OAB management. Improved education and qualitative management are required in the treatment of OAB, especially in non-urologists based on proper OAB treatment guidelines and prescriptions that should be verified by scientific value, merit, and defects of selected medication regimens to improve the quality of care for patients.



