Введение. Радикальная простатэктомия (РПЭ) является одним из основных методов лечения пациентов с клинически локализованным раком предстательной железы (РПЖ) [1]. Однако РПЭ может негативно влиять на сексуальную функцию и мочеиспускание, снижая качество жизни пациентов [2]. Нервосберегающая техника (НСТ) РПЭ позволяет минимизировать побочные эффекты лечения и обеспечивает условия для восстановления эректильной функции после операции [3]. Сохранение сосудисто-нервных пучков (СНП) рекомендуют пациентам с низким риском экстракапсулярной экстензии (ЭКЭ); в то же время некоторые исследователи указывают на относительную безопасность НСТ для отобранных больных РПЖ высокого риска и даже в случае спасительной РПЭ при рецидиве после первичного лечения [4, 5].
В настоящее время в литературе описаны различные варианты НСТ при РПЭ [6]. При этом любая техника сохранения перипростатической ткани потенциально увеличивает риск положительного хирургического края (ПХК) и, соответственно, биохимического рецидива РПЖ [7, 8].
Таким образом, принятие решения о сохранении СНП – поиск своеобразного баланса между необходимой онкологической эффективностью и максимально возможным сохранением функции. Несмотря на ряд факторов и алгоритмов, предложенных в качестве отбора пациентов при планировании РПЭ с НСТ (данные мультипараметрической МРТ, номограммы и т.д.), сохранение СНП нередко сопровождается неблагоприятными патоморфологическими находками, способными в дальнейшем негативно влиять на онкологические результаты [9, 10].
Цель исследования: определение и сравнительная оценка онкологических рисков крупной современной серии РПЭ с НСТ, а также определение основных дальнейших направлений возможной оптимизации онкологической безопасности НСТ.
Материалы и методы. Проспективное нерандомизированное исследование выполнено на научно-клинической базе кафедры урологии и хирургической андрологии РМАНПО и клиники урологии ГКБ им. С. П. Боткина, представляло собой анализ проспективно наполняемой базы данных, содержащей пред-, интра- и послеоперационную информацию о результатах хирургического лечения пациентов с РПЖ за период с 2014 по 2018 г.
Группу исследования составили 313 пациентов с сохранной предоперационной эректильной функцией (≥18 баллов по данным анкетирования с использованием международного индекса эректильной функции), перенесших РПЭ с односторонним (n=148) или двусторонним (n=165) нервосбережением. Окончательное решение об использовании НСТ принималось индивидуально на основании клинических данных (уровень ПСА, результаты биопсии предстательной железы, данные пальцевого ректального исследования, мультипараметрической магнитно-резонансной томографии) и информированного выбора пациента. Контрольную группу составили 592 пациента с локализованным РПЖ (стадия cT1–2), которым была проведена РПЭ без сохранения СНП.
Оперативные вмешательства (робот-ассистированная РПЭ, позадилонная РПЭ) выполнялись двумя хирургами, при наличии показаний нервосбережение осуществляли с использованием техники частичного или полного сохранения СНП [11]. Робот-ассистированные операции проводили с помощью роботизированной хирургической системы da Vinci Si (Intuitive Surgical®) трансперитонеальным доступом.
Гистологическое исследование всех удаленных препаратов (предстательная железа с семенными пузырьками; при наличии – лимфатические узлы, отдельные участки дистальной уретры/шейки мочевого пузыря) проводили в патологоанатомическом отделении ГКБ им. С. П. Боткина. Наличие опухолевой ткани в окрашенном крае резекции определялось как положительный хирургический край (ПХК). Биохимический рецидив определяли как повышение уровня ПСА ≥0,2 нг/мл в двух последовательных измерениях или как начало адъювантной лучевой или гормональной терапии. Безрецидивную выживаемость оценивали у пациентов с минимальным периодом наблюдения 12 мес. с момента операции.
Статистический анализ. Статистическую обработку данных проводили с помощью программного обеспечения Graph Pad Prism 8 (Graph Pad Software Inc, LaJolla, США). Для оценки непрерывных переменных использовали U-тест Манна–Уитни, при сравнительном анализе категориальных данных – тест χ2 (χ2 Пирсона). Безрецидивную выживаемость оценивали с помощью метода Каплана–Мейера, для сравнительного анализа выживаемости использовали лог-ранк тест. Во всех случаях за статистически значимое принимали значение p<0,05.
Результаты. Клинические характеристики групп представлены в табл. 1. По сравнению с контрольной группой пациенты группы исследования были ожидаемо моложе, имели более низкий уровень ПСА, большинство имели локализованный РПЖ низкой группы риска. Робот-ассистированный доступ в группе РПЭ с НСТ был основным, в то время как в группе РПЭ без НСТ оперативные вмешательства робот-ассистированным и позадилонным доступом выполняли приблизительно в равном соотношении.
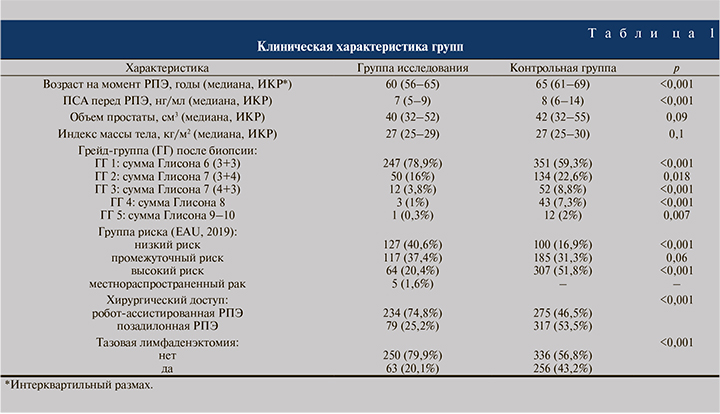
Неблагоприятные патоморфологические находки встречались в группе исследования значительно реже, чем в контрольной (табл. 2): ЭКЭ имела место в 9,4 и 18,75% случаев (p<0,001), повышение ГГ – в 23 и 29,3% (p=0,04), ПХК – в 15 и 22,1% соответственно (p=0,01).

При дополнительной оценке в зависимости от группы риска оказалось, что частота ПХК при высоком риске существенно ниже в группе исследования (15,6 против 30,3% в контрольной группе, p=0,017). При низком риске отмечена обратная тенденция, однако различия не достигли статистической значимости (12,6 против 7%, p=0,16). Частота ПХК при одно- и двусторонней НСТ составила 16,2 и 13,9% соответственно (p=0,54). При этом в случае односторонней НСТ у 10,8% пациентов ПХК обнаружен со стороны сохранения СНП, у оставшихся 5,4% – с контралатеральной стороны.
При дополнительном анализе группы исследования частота ПХК достоверно не различалась в зависимости от хирургического доступа: 13,9% (11/79) и 15,4% (36/234) (p=0,75). Положительный хирургический край в группе исследования в апексе локализовался в 5,8% (18) случаев, в области боковой поверхности – в 4,2% (13), в базисе – в 3,5% (11), в шейке мочевого пузыря – в 0,9% (3). Мультифокальный ПХК имел место в 0,6% (2) наблюдений.
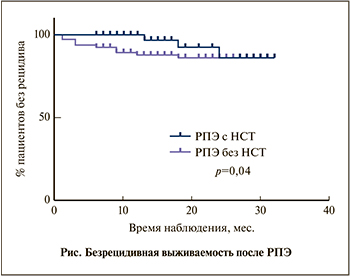 Медиана наблюдения в группе исследования составила 16 мес. (n=57), в контрольной группе – 18 мес. (n=117).
Медиана наблюдения в группе исследования составила 16 мес. (n=57), в контрольной группе – 18 мес. (n=117).
В целом биохимический рецидив отмечен у 5,3% (3/57) пациентов группы исследования и у 12% (14/117) – контрольной группы, а безрецидивная выживаемость через 12 мес. составила 100 и 88,2%, через 20 мес. – 92,3 и 86,4% соответственно (см. рисунок, p=0,04).
Обсуждение. Вопрос возможного негативного влияния НСТ на онкологическую безопасность РПЭ остается предметом дискуссий. Так, M.A. Preston et al. [7] указали на повышенный риск ПХК при билатеральном сохранении СНП у пациентов со стадией pT2 (но не pT3), а также на большую частоту ПХК при выполнении РПЭ с НСТ робот-ассистированным доступом. С другой стороны, системный обзор и мета-анализ [12] продемонстрировали отсутствие повышения риска ПХК при нервосбережении на стадиях pT2 и pT3.
Наше исследование показало в целом более низкую частоту ПХК в группе НСТ, однако наиболее интересным представляется дополнительная оценка обнаружения данного признака в зависимости от группы риска. В случае локализованного РПЖ высокого риска ПХК при РПЭ с сохранением СНП встречался практически в 2 раза реже по сравнению с контрольной группой. Схожие результаты получены в исследовании [13]: 35,5% без сохранения НСТ и 12,1% после операций, не затрагивающих СНП. Данную особенность, по всей видимости, можно объяснить тщательным отбором больных и более неблагоприятными клинико-морфологическими характеристиками пациентов высокого риска в контрольной группе. В то же время при РПЖ низкого риска отмечена тенденция к повышению частоты ПХК в группе исследования. Причиной могут быть как повышенные риски ятрогенного внедрения в ткань железы при сохранении СНП, так и недостаточно эффективный прогноз возможной ЭКЭ со стороны нервосбережения. По данным ряда отечественных [14] и иностранных авторов [15], занижение стадии распространенности и дифференцировки опухоли встречается очень часто (36,2–81,3 и 51,5% соответственно).
Одним из основных прогностических инструментов, способных оптимизировать отбор пациентов и выявлять потенциальные неблагоприятные характеристики заболевания, является мультипараметрическая МРТ (мпМРТ). На данный момент непосредственный опыт клинического применения продемонстрировал умеренные позитивные результаты. Так, R. Schiavina et al. [16] отметили, что анализ результатов мпМРТ перед оперативным вмешательством привел к изменению хирургической тактики в отношении сохранения СНП в 46,7% случаев и позволил снизить частоту ПХК по сравнению с контрольной группой с 24,1 до 12,4%. В исследовании S. S. Druskin et al. [17] по результатам предоперационного выполнения мпМРТ отмечены схожие тенденции, однако различия не достигли статистической значимости. Ранее мы продемонстрировали прогностическую значимость измеряемого коэффициента диффузии в отношении определения возможного повышения степени злокачественности РПЖ после РПЭ [18]. В настоящее время результаты мпМРТ внедряются и в алгоритмы отбора пациентов при планировании НСТ: A. Martini et al. [19] и J. Nyarangi-Dix et al. [20] предложили номограммы для прогнозирования ЭКЭ. Разработанный A. Martini et al. [21] алгоритм выбора варианта НСТ на основании собственной номограммы показал целесообразность при ретроспективном анализе, однако его дальнейшее использование зависит от результатов будущих проспективных исследований по оценке его реальной клинической эффективности.
Существенно снизить частоту ПХК при НСТ может методика срочного гистологического исследования замороженных срезов участков простаты, прилегающих к СНП. Протокол NeuroSAFE был впервые предложен T. Schlomm et al. [22] в 2012 г., а его применение позволило чаще использовать НСТ (особенно при РПЖ высокого риска) и снизить частоту ПХК, в том числе в проспективных исследованиях при валидизации в других клиниках [23].
К настоящему моменту предложены различные технические модификации методики [24], а наш собственный первый опыт ее клинического использования продемонстрировал оптимистичные предварительные результаты. Другим интересным направлением минимизации онкологических рисков РПЭ с НСТ являются методики интраоперационной визуализации, из которых наиболее перспективными представляются оптическая когерентная томография [25] и конфокальная лазерная эндомикроскопия [26].
Заключение. Радикальная простатэктомия с нервосбережением не компрометирует онкологическую безопасность, характеризуется более благоприятными патоморфологическими исходами и показателями безрецидивной выживаемости по сравнению с результатами лечения пациентов с локализованным РПЖ, перенесших экстрафасциальную РПЭ с иссечением СНП. В то же время полученные результаты в целом не оптимальные и указывают на необходимость дальнейшего совершенствования алгоритмов планирования и методик интраоперационного контроля качества хирургического лечения.



