В структуре бактериальных инфекций у женщин инфекции мочевыводящих путей (ИМП) занимают второе место, уступая лишь инфекциям дыхательных путей, при этом более чем у 35% женщин после первого эпизода ИМП в течение ближайших 6 мес. отмечается рецидив заболевания. В большинстве (80%) случаев эти инфекции имеют неосложненный характер (нИМП) [1]. К наиболее распространенным симптомам нИМП относят частое мочеиспускание, неотложные позывы на мочеиспускание, дизурию и боль в надлобковой области. Неосложненные ИМП характеризируются отсутствием структурных и функциональных изменений со стороны мочевыводящих путей, тогда как заболевания почек и коморбидный фон повышают риск осложнений ИМП. В исследовании [2] спонтанное излечение 28% пациенток наблюдалось в ходе первой недели, еще 37% – в течение последующих 5–7 нед. Тем не менее полученные данные свидетельствуют о недостаточной эффективности альтернативных методов лечения и возможности самоизлечения при нИМП.
Наиболее частой уропатогенной флорой при нИМП являются E. coli, ряд бактерий семейства Staphylococcaceae, Enterococcaceae и Enterobacteriacea [3]. При этом индивидуальная восприимчивость организма к инфекциям определяется не только патогенностью микроорганизмов, но и состоянием иммунной системы [4]. Адаптивный иммунный ответ развивается медленно, так как предполагает поэтапную активацию и пролиферацию Т- и В-лимфоцитов. Более быстрый ответ обеспечивается врожденным иммунитетом, в частности специальными рецепторами, к которым в первую очередь относятся toll-подобные рецепторы (toll-like-receptor, TLR). Эти последние широко представлены в клетках макроорганизма, распознают и взаимодействуют с молекулярными структурами, общими для целых групп патогенов [5]. TLR относятся к семейству паттернраспознающих рецепторов, осуществляют распознавание молекулярных структур патогенов и ряда эндогенных лигандов, обеспечивая быструю реакцию клетки.
После связывания лиганда TLR запускают сигнальный каскад с вовлечением ряда адаптерных белков, что ведет к активации ядерных факторов, последующей продукции цитокинов и прочих молекул, ассоциированных с воспалением. TLR-сигнальный путь контролируется посредством различных механизмов обратной связи. TLR служат предметом активного изучения как в норме, так и при различных патологических состояниях органов мочевыделительной системы, в том числе при онкологических процессах (рис. 1) [7].
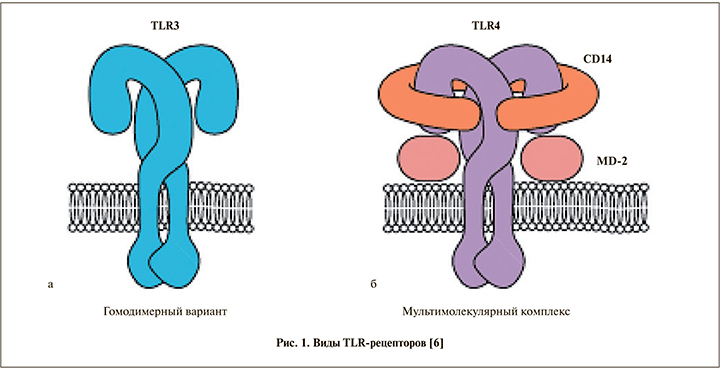
Помимо иммунного ответа, по мнению D. J. Sellers et al., TLR принадлежит ключевая роль в развитии болевого синдрома при ИМП, в том числе при интерстициальном цистите [8]. В проведенном авторами исследовании на группах мышей, у которых цистит был моделирован при помощи циклофосфамида, показано, что блокада TLR4 позволяет эффективно бороться с болевым синдромом. Роль TLR в иммунном ответе при инфицировании мочевыводящих путей была также оценена C. B. Ching et al. в исследовании передачи уровней интерлейкина-6 (ИЛ-6) и Stat3 [9]. Было показано, что исследуемые параметры коррелируют с тяжестью ИМП, однако специфический вклад ИЛ-6 в иммунный ответ хозяина при адгезии бактериальных уропатогенов по-прежнему не известен. В условиях in vivo на группе мышей [8], у которых цистит был моделирован при помощи тамоксифена, трансуретральная инокуляция уропатогенной E. coli приводила к секреции ИЛ-6, фосфорилированию уротелиального Stat3 и активации транскрипции антимикробного пептида при помощи TLR4 (рис. 2).
 Причем рекомбинантный ИЛ-6 вызывал фосфорилирование Stat3 в первичных уротелиальных клетках in vitro, тогда как системное введение ИЛ-6 способствовало фосфорилированию уротелиального Stat3 и экспрессии антимикробных пептидов in vivo. Дефицит ИЛ-6 приводил к снижению фосфорилирования уротелия Stat3 и экспрессии мРНК антимикробного пептида на фоне ИМП, что подтверждалось условной делецией Stat3. Дефицит ИЛ-6 или Stat3 был связан с повышенным образованием внутриклеточных бактериальных частиц, что in vivo приводило к тяжелому пиелонефриту у мышей. Авторами был сделан вывод о взаимосвязи уровня ИЛ-6/Stat3 в транскрипционной экспрессии антимикробного гена в инфицированном уротелии [9].
Причем рекомбинантный ИЛ-6 вызывал фосфорилирование Stat3 в первичных уротелиальных клетках in vitro, тогда как системное введение ИЛ-6 способствовало фосфорилированию уротелиального Stat3 и экспрессии антимикробных пептидов in vivo. Дефицит ИЛ-6 приводил к снижению фосфорилирования уротелия Stat3 и экспрессии мРНК антимикробного пептида на фоне ИМП, что подтверждалось условной делецией Stat3. Дефицит ИЛ-6 или Stat3 был связан с повышенным образованием внутриклеточных бактериальных частиц, что in vivo приводило к тяжелому пиелонефриту у мышей. Авторами был сделан вывод о взаимосвязи уровня ИЛ-6/Stat3 в транскрипционной экспрессии антимикробного гена в инфицированном уротелии [9].
Обзор литературы показал, что наряду с TLR бактериофаги также могут вызывать иммунный ответ, способствуя поддержанию иммунного гомеостаза, тем самым сдерживая распространение бактериальной обсемененности [10]. Фаги могут уменьшать активацию Т-клеток, а также выработку иммуноглобулина in vitro и общую аутоиммунную реакцию. Степень воспалительной инфильтрации, вызванной эндотоксином, также может быть заметно снижена путем введения бактериофагов [11, 12]. Объем накопленных данных свидетельствует о том, что бактериофаги могут быть полезными при иммунотерапии различных ИМП [13, 14]. Данная гипотеза подтверждается главным образом тем фактом, что фаги не влияют на способность гранулоцитов и моноцитов уничтожать бактерии. Снижение количества патогенных и условно патогенных бактерий при использовании фагов может быть объяснено концепцией «синергии иммунофагов» – совместного действия фагов и фагоцитов [15, 16].
Как именно бактериофаги взаимодействуют с макроорганизмом и насколько сильно их влияние на иммунный ответ, остается плохо изученным. По мнению A. Sinha et al. [17], развитие гуморального иммунного ответа в ответ на применение фаговой терапии возможно за счет бактериально-опосредованного воздействия последних на клетки макроорганизма. В развитии иммунитета против бактериальной ИМП наиболее изучена роль нитевидного бактериофага (Pf), продуцируемого Pseudomonas aeruginosa. Всемирная организация здравоохранения недавно классифицировала P. aeruginosa как «приоритетный патоген», представляющий наибольшую опасность для человека [18]. По данным J. M. Sweere et al. [19], лейкоциты поглощают Pf, а интернализация одноцепочечного ДНК-вируса приводит к образованию фаговой РНК. Это запускает TLR-3 и интерферон β (интерферон I типа), а также ингибирует фактор некроза опухолей (TNF) и подавляет фагоцитоз. В серии экспериментальных исследований авторами показано, что иммунизация мышей против Pf предотвращает развитие инфекции, ассоциированной с P. aeruginosa. Таким образом, присутствие Pf в организме запускает врожденные иммунные реакции, которые снижают бактериальную обсемененность. К подобным выводам пришли P. R. Secor et al. [20], доказавшие в исследовании на культурах белых мышей эффективность Pf в ингибировании бактериальной инвазии эпителиальных клеток, снижении уровня нейтрофилов и цитокинов.
Более того, в случае продуцирования Pf-фага P. aeruginosa была менее склонна к фагоцитозу макрофагами по сравнению с бактериями, не продуцирующими Pf-фаг. Вместе с тем в той же работе авторы высказали предположение о появлении фенотипов Pf, опосредованно влияющих на фагоцитоз. Исследования in vivo показали, что Pf может быть одной из причин хронизации воспалительного процесса за счет увеличения защитных механизмов P. aerugi-nosa.
В формировании защитного механизма бактерий принимает участие CRISPR-система (clustered regularly interspaced short palindromic repeats) – особые локусы бактерий и архей, состоящие из прямых повторяющихся последовательностей, разделенных уникальными последовательностями (спейсерами) [21]. Спейсер представляет собой короткий участок вирусной или плазмидной ДНК. Размер CRISPR-повтора исчисляется 23–47 нуклеотидными парами, а спейсеров – от 21 до 72 нуклеотидных пар. Число групп повтор/спейсер может достигать 375, но обычно не превышает 50. В бактериальном геноме может быть не один, а несколько локусов CRISPR. В непосредственной близости от CRISPR расположены гены специальных белков, называемые Cas (CRISPR associated). Обычно Cas – это нуклеазы, полимеразы, нуклеотидсвязывающие белки; всего эта группа объединяет около 40 семейств белков [21]. В ответ на одну и ту же вирусную инфекцию даже генетически идентичные бактериальные клетки вставляют в свой геном разные спейсеры, соответствующие разным участкам генома вируса. Данный факт в свою очередь уменьшает активность TLR, снижая иммунный ответ.
Проведя секвенирование генома фага ICP1, K. D. Seed et al. [23] пришли к следующему выводу: исследуемый фаг содержит полный набор генов системы CRISPR. Это исследование легло в основу дальнейшего изучения роли фаготерапии в развитии иммунного ответа. И если применение фаготерапии в той или степени позволяет воздействовать на CRISPR бактериальной клетки, способствуя развитию иммунного ответа, то возникновение дефектов на уровне TLR полностью нивелирует преимущество фагов и может обусловливать развитие инфекционных и воспалительных заболеваний. К основным причинам нарушения функционирования TLR могут быть отнесены мутации в генах и факторах системы передачи сигнала с TLR, а также полиморфизм генов, кодирующих TLR [24]. Так, например, мутации гена TLR4 (Asp299Gly и Thr399Ile), с которыми связывают отсутствие адекватного иммунного ответа на липополисахарды бактериальной стенки грамотрицательных бактерий (в исследованиях in vivo и in vitro), могут быть причиной сепсиса [25].
Проведенный обзор работ по изучению экспрессии и функционирования TLR показал, что большая часть из них носит фрагментарный характер, что предопределяет дальнейшее изучение данного вопроса. Определение роли TLR в развитии иммунного ответа при инфекциях органов мочевыделительной системы позволит в равной степени прогнозировать течение заболевания и значительно повысит эффективность проводимой терапии. Учитывая высокую частоту рецидивных форм ИМП, а также неуклонный рост числа резистентных антибактериальных штаммов, все большую актуальность приобретают как оптимизация рационального использования противомикробных препаратов, так и разработка альтернативных методов лечения. Внедрение в клиническую практику фаговой терапии позволит эффективнее бороться с имеющимся воспалением за счет активного воздействия на CRISPR бактериальной клетки и как следствие – на уровне TLR.



